Crystalline and Amorphous Solids
Solid is one of the fundamental states of matter. Most things surrounding us are solids. Solids are widely used for different applications. Solids are rigid and generally compressed, and tightly connected through chemical bonds.
Characteristics of Solids:
- They have definite mass, volume, and shapes.
- Small intermolecular distances.
- Strong intermolecular force.
- Incompressible.
- Occupy ample space.
Difference between Amorphous Solid and Crystalline Solid:
| Crystalline Solids |
Amorphous solids |
- Regular/geometric shape.
- Anisotropic in nature.
- Sharp and characteristic temperature range, which means the transition from solid to liquid state suddenly happens over the fixed value of temperature, not over a range.
- True solids.
- They have well-resolved X-ray diffraction.
|
- Irregular shape.
- Isotropic in nature.
- Soften gradually over a range of temperatures.
- Pseudo solids.
- They do not have well-resolved X-ray diffraction.
|
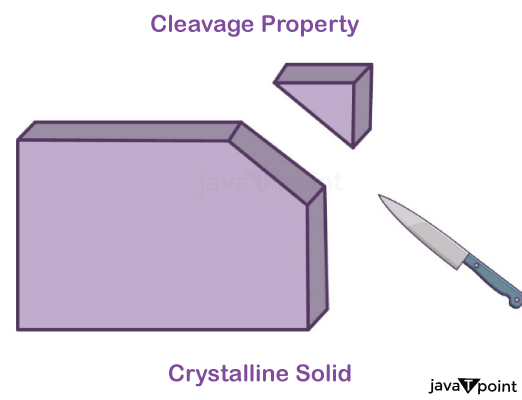
Properties of Crystalline Solid:
- Crystalline solids are orderly arranged and practically incompressible.
- Crystalline solids have a definite melting point.
- The crystalline solids can be further classified into single and polycrystalline solids.
- Polycrystalline solids contain many small single crystals.
- Crystalline solids have different physical properties and directions; this phenomenon is called
- In polycrystalline solid regular order exist only over a small region of the Crystal.
- Newly generated surfaces are plain and smooth.
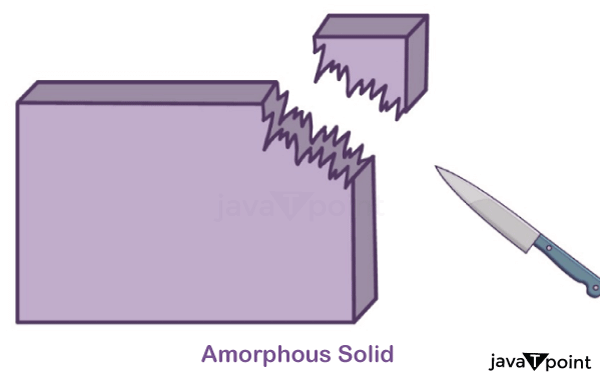
Properties of Amorphous Solids:
- Amorphous solids do not have long-range order, which means they have an irregular arrangement of particles. This is the most notable property of amorphous solids.
- The physical properties of amorphous solids are the same in all directions.
- Due to the lack of regular shape, amorphous solids have variable densities.
- Amorphous solids undergo a process of crystallization. Under specific conditions, they undergo a transition to a crystalline solid.
- Amorphous solids have various applications, including-glass manufacturing, amorphous metal, and amorphous Polymers.
- Newly generated surface is irregular in shape.
Classification of Crystalline Solids
Crystalline solids are further classified based on intermolecular force-
- Molecular solids.
- Ionic solids.
- Metallic solids.
- Covalent solids.
1) Molecular Solids:
Molecular solids are classified into three types.
- Non-polar Molecular Solids: In nonpolar molecular solids, molecules are held together by weak forces. Non-polar molecular solids have an unsymmetrical distribution of electrons. At room temperature and pressure, non-polar solids exist in a liquid and gaseous state.
- Polar Molecular Solids: Relatively strong forces like dipole-dipole interactions hold molecules in polar solids together. Polar molecules solids do not conduct electricity. Compared with non-polar molecule solids, polar molecules solids have high melting points yet are in a liquid or gas state under room temperature and pressure.
- Hydrogen-Bonded Molecular Solids: Hydrogen bonding is a relatively strong, highly directional, and specific noncovalent interaction present in any atoms, molecules, or organic molecules. Such types of solids are known as hydrogen-bonded molecular solids. They do not conduct electricity. A hydrogen bond is also seen between atoms of H2O molecules. Unlike non-polar and polar molecules, they are complex.
2) Ionic Solid:
Ionic solids are formed by the three-dimensional arrangement of cations and anions held together by electrostatic forces.
Some of the properties of ionic solids are-
- They are hard.
- They are brittle (can be made in sheets).
- They have a high melting point.
- They have a high boiling point.
- They behave like insulators.
- The molten state of ionic solids conducts electricity.
3) Metallic Solid:
Metallic solid is a compound made up of metal atoms held together by metallic bonds. Metallic solids are made up of free atoms and free-flowing electrons, known as a sea of delocalized electrons. Metallic solids have many characteristics and features-
- They are lustrous.
- They conduct electricity (due to the presence of free electrons).
- Metallic solids are highly malleable and ductile.
- They have high thermal energy.
4) Covalent Solids:
Various types of crystalline solids result from forming covalent bonds. Covalent solids are solid and have a complex network of atoms. Covalent solids are also known as giant molecules and network solids. Covalent solids have high melting points. Diamond, graphite, and silicon carbide are examples of covalent solids. Graphite has a giant, complex structure in which each carbon atom is covalently bonded to three other carbon atoms.
Lattice:
A lattice is a regular imaginary point representing how atoms, ions, or molecules are arranged in space. Thus, a regular three-dimensional arrangement of particles is known as a crystal or space lattice.
There are 14 three-dimensional lattices known as Bravais lattices. The minor portion of a lattice is known as a unit cell. Unit cells are the majority of two types-
Unit cell: The unit cell is the smallest portion of the crystal lattice which, when repeated in a different direction, generates the entire lattice.
- Primitive unit cell: When constituent particles are present only in the corner positions of the unit cell, it is called the primitive unit cell.
- Centered unit cell: When a unit cell contains one or more constituent particles at corners and other positions like edges and center, it is called as center unit cell. Center unit cell is further classified into three categories:
- Body-centered unit cell: In such unit cells, one constituent particle is at its body center besides the ones that are at its corners.
- Face-centered unit cell: In such unit cell, one constituent particle is at the center of each face besides the one that are present at its corners.
- End-centered unit cell: In such unit cell, one constituent particle is present at the center of any two opposite faces besides the one at its corners.
Solids have numerous applications, from a fundamental thing like a pen to a complex structured substance like a diamond are solid. The Internal structure is used as a primary criterion for the classification of new solids.
|



 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now









