Concentration of SolutionsThe amount of solute present or dissolved in a solution is called the concentration of that solution. The amount of solute or solvent is not fixed in a solution. So, based on the proportion of solute present in a solution, a solution can be of two types; 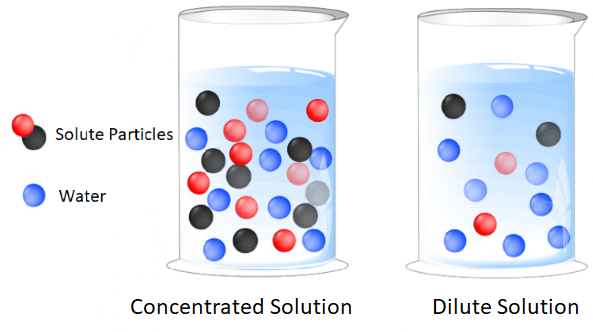
i) Dilute solution: A solution in which less amount of solute is present in the solvent is called a dilute solution. It can be diluted further by increasing the quantity of solvent. ii) Concentrated solution: It contains a large amount of solute or the concentration of solute is more. Besides this, it can become more concentrated if the amount of solute increases. For example, one tablespoon of sugar in 500 ml of water can be considered a dilute solution when we compare it with a solution made by dissolving 10 tablespoons of sugar in 500 ml of water. In other words, we can say that the more is the solute, the more will be the concentration. For example, 5 tablespoons of salt in 300 ml of water will be saltier than one tablespoon of salt in 300 ml of water. Dilute or concentrated expresses the concentration of solutions qualitatively as we can only say that a solution is either dilute or concentrated, but cannot tell the exact concentration. So, it is not useful in chemistry. There are many methods or ways to express the concentration of solutions quantitatively. These methods tell the exact concentration of the solutions that make it easy to compare the concentration of two solutions. Let us take different types of solutions one by one and see how is their concentration expressed or measured quantitatively. 1) Solid in liquid solutionThe concentration of a solution made of solid solute and a liquid solvent is expressed in mass by mass percentage (mass percentage) or mass by volume percentage. In the numerator, we write the mass of solute and in the denominator, we write the total mass of the solution (mass of solute + mass of solvent) and multiply it by 100 as explained below; i) Mass percentage or Mass by mass percentage (w/w):It refers to the mass of solute in grams dissolved in 100 grams of the solution. It tells the percentage of solute by mass in solution. For example, a 20 percent solution of sugar means 20 gm of sugar is dissolved in 100 gm of the solution (20 gm sugar + 80 gm of solvent). Similarly, a 10% mass by mass solution of common salt, indicates that 10 gm of common salt is added to 90 gm of the water so that the total mass of solution becomes 100 grams including 10gm of common salt dissolved in it. So, it indicates the mass of solute in 100 grams of the solution, not in 100 grams solvent. The formula to express the concentration in mass percentage is as follows; Mass percentage = (Mass of solute / Mass of solution in grams) x 100 %
The above formula tells the grams of solute per 100 grams of the solution. So, when the solute is a solid and solvent is liquid, the mass percentage is a suitable way to express the concentration. For example; when we prepare a solution by dissolving 20 grams of sugar into 100 grams of water; Mass percentage or percentage by mass = (20 gm of sugar / 120 gm sol.) x 100 % = 16.6 % sugar ii) Mass by volume percentage (w/v):It refers to the mass of the solute in grams dissolved in 100 millilitres (ml) of the solution. For example, a 10 % mass by volume solution of common salt in water means 10 grams of common salt is dissolved in 100 ml of the solution. The formula to measure the mass by volume percentage of a solute is as follows; Mass by volume percentage of a solute = (mass of solute in grams / volume of the solution in ml) x 100 Concentration of very dilute solutionsThere are certain cases when the amount of solute is very small as compared to the solvent. In these cases, the concentration is expressed in parts per million (ppm). It refers to the parts of solute by mass or volume per million parts of the solution by mass or volume. Mathematically, we can write it as follows; ppm = mass of solute/total mass of solution x 106 ppm = volume of solute / volume of solution x 106 2) Liquid in liquid solutionsIn this type of solutions, both solute and solvent are present in the liquid state. In this case, the concentration of the solution is expressed in volume by volume percentage or volume percentage of the solution, i.e. the percent of solute in the solution by volume. Volume percentage (v/v) of solute:It is used for liquid in liquid solutions. For example, the volume of solute in millilitres (ml) dissolved in 100 millilitres (ml) of the solution. The formula to calculate the concentration of these solutions is as follows; Vol. percentage or Vol. by vol. percentage = (vol. of solute in ml / vol. of sol. in ml) x 100 % So, a 20% volume by volume solution of alcohol in water is made of 20 ml of alcohol and 80 ml of water so that the total volume of the solution becomes 100 ml as shown below; Vol. percentage = (20 ml alcohol / 100 ml of sol.) x 100 % = 20 % The concentration of medicines in liquid form is also expressed in volume by volume percentage and is denoted by the symbol v/v. So, when the volumes of the solute and the solution are given, the concentration of the solution is expressed in volume percent. Other popular methods:i) Molarity (M)It refers to the number of moles of solute dissolved in one litre of the solution. It is calculated by dividing the no. of moles of solute by the volume of the solution in litres as shown below in its formula; Molarity (M) = (moles of solute / litres of solution) = moles / Litre
The unit of Molarity is mol/L or mol/dm3 or symbol M, which is read as 'molar'. For example, a solution that is labelled as 2.5 M NH4Cl is read as '2.5 molar ammonium chloride solution'. The number of moles of a substance or solute can be calculated by using the following formula; No. of moles = given mass of the substance / molecular mass of the substance Furthermore, molarity tends to change with temperature as volume changes with a change in temperature. ii) Molality (m)It also expresses the concentration of a solution. It tells the number of moles of solute present or dissolved per kg of the solvent. It is denoted by 'm' and its formula is as follows; Molality (m): (Moles of solute / Mass of solvent in kg) = mol / kg
The unit used for molality is mol/kg. So, a solution whose molality is 7 mol/kg is said to be 6 molal or 6 m. Molality is different from molarity only in the denominator as in molarity we have litres of sol. whereas in molality we have kilograms of solvent in the denominator. The value of the molality does not change with a change in temperature. iii) Normality (N)It is defined as the number of gram-equivalents of the solute present in one litre or one cubic decimetre of the solution. It is also called the equivalent concentration. It is denoted by 'N' and its unit is equivalent per litre or eq/L. A solution whose normality is one is called a normal solution. Its formula is as follows; Normality (N) = (weight or mass of solute in grams / equivalent weight or mass x vol. in litre)
iv) FormalityIt is defined as the number of gram formula masses of an ionic solute dissolved in one litre of the solution. It is denoted by F. For example, the formality of a solution will be 1 when it contains one gram formula mass of solute per litre of the solution. Formality is generally used to express the concentration of ionic solids like NaCl that exist as a network of ions. Furthermore, the formality changes if the temperature changes. Its formula is given below; Formality = Number of gram formula masses of solute / Vol. of sol. in litres.
= mass of the solute in gram / gm. formula mass of solute x volume in litre Although both molarity and formality tell moles of solute in one litre of solution, they are different from each other. Let us see the basic difference between molarity and formality; Formality tells the total concentration of a substance in solution irrespective of its chemical form. For example, when we dissolve 0.2 mol of NaCl in 1 L of water, it results in a solution containing 0.2 mol of Na+ and 0.2 mol of Cl-. So, the molarity of NaCl is zero as there are no un-dissociated NaCl molecules in the solution. However, this solution is 0.1 M in Na+ and 0.1 M in Cl-. But, the formality of NaCl in this solution is still 0.1 F. v) Mole FractionIt refers to the ratio of moles of a component to the total moles (moles of all components) present in the solution. It is denoted by x. For example, a solution contains solute A in solvent B. Let p is the no. of moles of solute A and q is the no. of moles of B. So, the mole fraction of these two components A and B is given as follows; xA = p / p + q xB = q / p+ q All the methods described above are commonly used to express the concentration of solutions. They have their own advantages and drawbacks. For example, molarity depends on temperature, whereas, the molarity and mole fraction are not affected by the temperature.
Next TopicColloidal Solutions
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










