Compound
A compound is a pure substance that is made of identical molecules formed of different elements in a fixed proportion. Compounds are considered pure as the constituting particles or their molecules contains two or more specific elements or atoms of elements in a fixed ratio. We can also say that the same type of molecules combines to form compounds, so, they are called pure substances. Whereas, if different molecules form a substance, we cannot call it a compound. In this case, it is called a mixture.
For example, water molecule in water is made of 2 hydrogen atoms and one oxygen atom combined in a fixed ratio and is represented by H2O, similarly, carbon dioxide molecule contains one carbon atom and two oxygen atoms in a fixed ratio, e.g. CO2,. Similarly, we have other molecules like carbon monoxide (CO), ammonia (NH3) and methane (CH4).
If the atoms do not combine in a fixed ratio they will not form molecules of a compound. The molecule of water always has 2 H atoms and 1 O atom. If hydrogen and oxygen atom combine in a different ratio, they will not form water molecules.
So, every compound is made of molecules and the atoms that make a molecule are always present in a fixed proportion in the molecule.
Compounds are different from molecules, some of the major differences between them are listed below;
Difference between molecule and compound
| Molecule |
Compound |
| It is made of two or more same or different types of atoms bonded together in a fixed proportion. |
It is made of the same type of molecules. |
| The constituting particles of molecules are atoms. |
The constituting particles of compounds are molecules. |
| All molecules are not compounds. For example, O2 is a molecule not a compound. |
All compounds are groups of molecules held together through the force of attraction. |
| Example, oxygen (O2), nitrogen (N2), ozone (O3), carbon dioxide (CO2), sulphuric acid (H2SO4). |
Example, common salt (sodium chloride or NaCl). |
| It cannot be seen with naked eyes. |
It can be seen with naked eyes. |
| It is a group of atoms. |
It is a matter in complete shape such as table salt, sugar cubes, etc. |
Properties of Compounds
- They are made of two or more different elements chemically combined in a fixed ratio.
- Compounds are formed as a result of a chemical change or reaction between elements and its properties are entirely different from its constituting particles or elements. For example, when we simply mix iron filings and sulphur powder, we get a mixture whose properties are the same as that of its constituting particles iron and sulphur. Whereas, when the mixture is heated a chemical reaction occurs and a new compound called iron sulphate is formed. It shows different properties than its constituents iron and sulphur.
- They can be separated into their constituting particles by chemical methods like electrolysis. They cannot be separated into their constituents by simple physical means. For example, water cannot be separated into hydrogen or oxygen through physical means like filtration, evaporation, distillation, solvent extraction or using a magnet.
- When a compound is formed, energy is generally evolved or absorbed. For example, when an electric current is passed to hydrogen and oxygen energy is absorbed and it forms water. During the formation of carbon dioxide (CO2) when coke is burned in the air or oxygen heat is evolved.
- A compound has a definite molecular formula and fixed boiling and melting point.
- The compound is a homogenous substance. For example, when a sample of iron sulphate is seen under a magnifying glass or a microscope, all the particles of iron sulphide are the same throughout the sample.
Types of compounds
Compounds are made of chemically bonded molecules. So, they are chemical compounds, which can be divided into two types that include ionic compounds and covalent compounds.
i) Ionic Compounds
As the name suggests, the chemical compounds whose molecules are held together through ionic bonds are called ionic compounds. They are also called electrovalent compounds. In ionic bonds, the transfer of electrons takes place from one element to another element. The element that prefers to lose electrons to complete its octet or stability lose electrons and the element who prefer to gain electrons to complete its octet or gain stability gains the electrons.
The atom which loses electrons becomes a cation (positively charged ion), whereas, the atom that takes those electrons become anion (negatively charged ion). So, we can say that ionic compounds are solids that are made of oppositely charged ions.
Ionic compounds are generally formed when metals react with non-metals. The metals have a tendency to lose electrons and thus to become a cation with a net positive charge. On the other hand, non-metals tend to gain electrons and thus tends to become anions with a net negative charge. So, chemical compounds are formed as a result of the transfer of electrons from one element to another element.
For example, in common salt, NaCl, the sodium (Na) and Chloride (Cl) atoms are held together by an ionic bond, a bond where one atom loses an electron and another atom gains the electrons.
Similarly, magnesium chloride forms an ionic compound as Magnesium with 2 valence electrons has a tendency to lose 2 electrons and Chlorine with 1 valence electron has a tendency to gain 1 electron. Here, Mg is losing two electrons so there will be two chlorine atoms. Thus, one Mg atom loses two electrons to 2 chlorine atoms to form an ionic compound.
Structure of Ionic Compounds
The oppositely charged ions form the ionic compounds, so, it is a collection of positively and negatively charged ions packed together in a regular and repeating structure called crystal lattice structure or ionic lattice. The smallest repeating unit of this structure is called the formula unit. It is the simplest ratio of oppositely charged ions that has no net electrical charge as the positive charge on cation is neutralized by the negative ion on the anion.
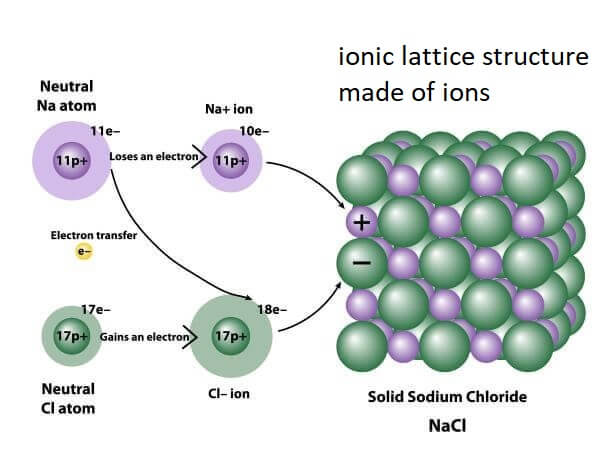
For example, in the ionic compound Sodium Chloride (NaCl), the sodium ions and chloride ions attract each other to form a 3-D (three-dimensional) structure of alternate Na+ and Cl- ions. These ions form an uncharged crystal of Sodium Chloride with no net charge as it has an equal number of sodium and chloride ions.
So, they have a huge lattice structure created by strong electrostatic forces of attraction between oppositely charged ions (anions and cations). The lattice structure expands in three dimensions that allows ionic compounds to form crystals with regular shapes. The lattice structure can be so huge that a single grain of salt may be made of 1.2 x 108 ions.
The ionic compounds can be oxides, hydroxides, salts, sulphides and most of the inorganic compounds. Furthermore, the structure of ionic compounds depends on oppositely charged ions that make the ionic lattice structure.
Properties of ionic compounds
- They are generally found in the solid state due to the strong force of attraction between cations and anions. However, they are brittle, so when pressure is applied they generally break into pieces.
- They are good conductor of electricity in the molten or aqueous state as they form ions, however, in the solid state they cannot conduct electricity because the ions are not free to move in the solid state because of the rigid structure in the solid state. So, when we heat an ionic compound to get in the molten state, the force of attraction between ions is overcome by heat and they become free to move and thus they can conduct electricity. Similarly, when we dissolve ionic compound in water, the force of attraction decreases and they split into ions which are free to move and thus can conduct electricity. So, in the molten or aqueous state, they break into free-floating ions that makes them good conductor of electricity.
- They have a high melting point and boiling point as a large amount of energy is required to break the strong force of attraction between the ions or to break the huge lattice structure held by the strong electrostatic force of attraction between oppositely charged ions. Only a large amount of energy can break the repeating pattern of ions.
- They are soluble only in polar solvents like water. They do not dissolve in other solvents which are non-polar such as kerosene, petrol, etc. The reason for their solubility in water is that they dissociated into ions when added into water. For example, sodium chloride (NaCl), sodium fluoride, ammonium nitrate dissociate into ions when dissolved in water. Due to this property, they are used in tooth pastes, fertilizers, etc.
- They have higher enthalpies of fusion and vaporization as compared to molecular compounds. Enthalpy of fusion is the heat required to melt a single mole of a solid under constant pressure. Whereas, the enthalpy of vaporization is the heat required to vaporize one mole of a liquid compound under constant pressure.
- Their ions are held together through ionic bonds which are formed when two oppositely charged ions attract each other by the electrostatic force of attraction.
- An ionic compound like sodium chloride common salt is used in food preparations and as an electrolyte.
Common Examples of Ionic Compounds
- Sodium Chloride (Nacl) is an ionic compound in which the ionic lattice structure is created by sodium and chloride ions, which are bonded together through ionic bonds.
- Magnesium Oxide, in which, the magnesium ion and oxide ion are held together through ionic bonds.
- Calcium Chloride, in which calcium and chloride ions are electrostatically attracted or bonded to each other to form the ionic lattice structure.
Similarly, other examples of ionic compounds include Sodium Bromide (NaBr), Potassium Bromide (KBr), Sodium Fluoride (NaF), Potassium Chloride (KCl), Potassium Oxide (K2O), Magnesium Oxide (MgO) and Potassium iodide (KI).
Naming ionic compounds?
There is a way to name the ionic compounds, the cation or atom with a positive charge is written first before the anion or atom with a negative charge. For example, NaCl (Na+ & Cl-). We can also say that the element symbol of metal is written first which is followed by the element symbol of the non-metal.
Covalent Compounds I Molecular Compounds
Covalent compounds are also known as molecular compounds. They are formed in a chemical reaction when two or more non-metal atoms bond together by sharing electrons between them. In covalent or molecular compounds, the non-metals atoms join together by a chemical bond called a covalent bond. The shared pair of electrons is called a covalent bond.
Structure of covalent compounds
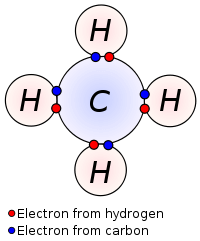
Ions are not formed in covalent compounds due to sharing of electrons between atoms and the absence of transfer of electrons. However, covalent compounds like sucrose can exist in lattice structure like ionic compounds exit, but the force of attraction is weaker in covalent than in ionic compounds. The force of attraction in covalent compounds is mainly dispersion forces including dipoles and hydrogen bonding in some cases.
Properties of covalent compounds
- They can exist as solid, liquids and gases at room temperature as they can have polar or non-polar covalent bonds. If they have polar covalent bonds such as in a molecule of water, they are mostly found in a solid or liquid state. If they have non-polar covalent bonds in their molecules, they are mostly found in a gaseous state. Furthermore, polar covalent compounds are harder than non-polar covalent compounds as molecules of polar covalent compounds are less free to move than the molecules of non-polar compounds.
- They have a low boiling point and melting point. However, the polar covalent compounds have more boiling or melting point than non-polar covalent compounds and are able to hold together a little bit longer before they turn into a liquid and turn from liquids into gases.
- They are soluble in non-polar liquids but rarely in water. The reason for this is that water is polar it has a slightly positive and slightly negative end. On the other hand, non-polar compounds have neutral areas around them and thus do not tend to come closer to charged areas so they tend to group together with other nonpolar compounds and tend to separate away from water such as oil and water do not mix. Oil has lots of nonpolar areas so it is more likely to come together and separate out away from the water. The polar covalent compounds might interact with water a little bit but not as strongly as the ionic compounds.
- They are poor conductor of electricity in all states of matter (solid, liquid, or gas) as they do not form ions that are required to move the electric charge. In covalent compounds, the atoms are held together and they do not change into ions, so there is nothing that can carry the charge.
- Most of the covalent compounds are flammable so they burn readily when heated. Mostly, the covalent compounds that are organic in nature burn readily as they contain carbon and hydrogen that react fast with oxygen at high temperatures. Not all covalent compounds burn, for example, water does not burn.
- They are also poor thermal conductors as heat does not move through them as their molecules are not strongly held as the ions in ionic compounds.
- They have a low enthalpy of fusion as it absorbs less heat when it is melted. They also have a low enthalpy of vaporization, i.e., the heat required to vaporize one mole of a liquid compound under constant pressure.
Common examples of covalent compounds
- Oxygen (O2)
- Chlorine (Cl2)
- PCl3 (phosphorus trichloride)
- Ethanol (CH3 CH2 OH)
- Ozone (O3)
- Methane (CH4)
- Proteins
- Lipids or fats
- Carbohydrates (CO2)
- Nucleic acids (DNA and RNA)
Difference between Ionic compound and covalent compound in tabular form
| Ionic Compounds |
Covalent Compounds |
| They are formed by the transfer of electrons, one atom loses electrons and the other atom gains electrons. |
They are formed by sharing of electrons between atoms. |
| They generally exist in the solid state. |
They generally exist in all states of matter such as solid, liquid and gas. |
| Ionic compounds generally have high melting and boiling point. |
They generally have low melting and boiling points. |
| They are soluble in water. |
Covalent compounds are less soluble in water. |
| Ionic compounds are good conductor of electricity in the molten state and in the form of aqueous solutions. |
Covalent compounds are bad conductor of electricity in the molten state and even in aqueous solution form. |
| The difference in the electronegativity of atoms forming the ionic compound is very high. |
The atoms forming the covalent compounds have almost the same electronegativity. |
| Ions are formed in an ionic compound. |
The formation of ions does not take place. |
| It is formed between a metal and a non-metal. |
They generally formed between non-metals. |
|


 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now









