Ammonium-IonAn ion is an atom or molecule with one or more positive or negative electrical charges. The ammonium ion is a polyatomic (ion containing more than one atom) cation with the chemical formula NH4+. The ammonium ion is an inorganic compound with positively charged tetrahedral nitrogen, as a part of its structure. The ammonium ion is formed by the protonation of ammonia (NH3). Protonation is the addition of a proton or hydrogen ion, usually denoted by H+. Four hydrogen atoms are bonded to one central nitrogen atom in ammonium ion. Ammonium ion carries a total charge of +1. 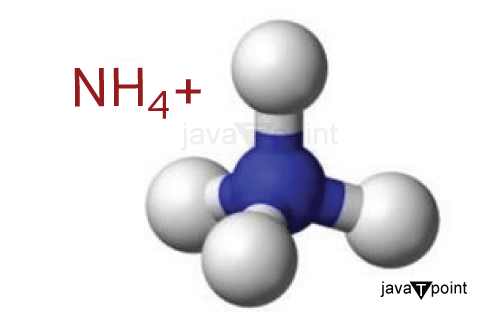
Formation of Ammonium IonsThe formation of ammonium ions involves the process of ammonia getting protonated by transferring hydrogen ions to its lone pair of electrons. In other words, an ammonium ion is formed when a neutral ammonia compound (NH3) is protonated or takes up an additional positively charged hydrogen atom. NH3 + H+ ------> NH4+ In ammonia, nitrogen is a central atom with 5 electrons in its valence shell, 3 of which are shared with 3 Hydrogen atoms, and 1 lone pair of electrons completes the valence shell configuration. The formation of ammonium ions from ammonia depends heavily on the pH of the solution. According to the pH of the solution, ammonia can produce the ammonium ion to varying degrees. When the pH is low, the equilibrium changes to the right, turning more ammonia molecules into ammonium ions. The equilibrium changes to the left if the pH is high (low hydrogen ion concentration, high hydroxide ion concentration), wherein the hydroxide ion removes a proton from the ammonium ion to produce water and ammonia gas. The formation of ammonium ions from ammonia and hydrogen ions forms a covalent coordinate bond. 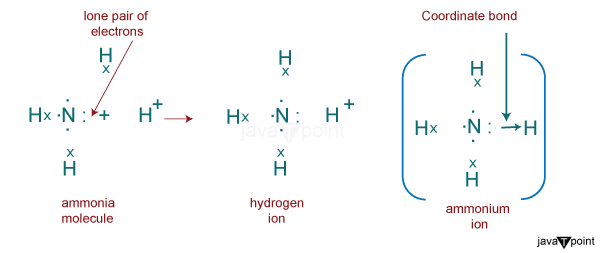
Bonding of Ammonium IonsCovalent bonds are formed when electrons are shared between elements that are non-metals. The ammonium ions have covalent bonds because both nitrogen and hydrogen are non-metals. Being polar covalent bonds, all four nitrogen hydrogen bonds are equivalent. Of these covalent bonds, one of them will be considered as a dative covalent bond. A dative covalent bond is formed when shared electrons come from the same atom. Therefore, ammonium ions have 3 ordinary covalent bonds and 1 dative covalent bond. Properties of Ammonium Ions
The Complex Reaction of Ammonium IonsAmmonium ions (NH4+) can participate in complex reactions by forming complexes with various metal ions. According to coordination chemistry, a complex is a structure consisting of a central metal ion or atom bonded to surrounding molecules or ions known as ligands. Ammonium ions form complexes with metal ions by donating lone-pair electrons: 1. Formation of Amine Complexes: Ammonium ions can coordinate with metal ions, such as transition metals, to form ammine complexes. For example, with copper (II) ions, [Cu (NH3)4]2+. The copper (II) ion is coordinated to four ammonia ligands in this complex. The ammonium ion donates its lone pair of electrons to form coordinate bonds with the copper ion. 2. Formation of Ammonia Borane Complexes: Ammonium ions can also form complexes with compounds like ammonia borane (NH3BH3). These complexes have applications in hydrogen storage and release. 3. Ion Exchange Reactions: Ammonium ions can undergo ion exchange reactions by replacing other cations in complexes. For example, in water treatment processes, ion exchange resins can be used to remove heavy metal ions from water by replacing them with ammonium ions. 4. Catalysis: Some catalytic reactions involve ammonium-containing ligands which coordinate with metal ions to facilitate specific chemical transformations. 5. Environmental and Analytical Chemistry: Ammonium ions can interact with metal ions in solution, affecting the solubility and speciation (the process of identification of different forms in which an element is present in a material) of metal ions. This has implications for environmental chemistry and analytical techniques. Applications of Ammonium Ions1. Agriculture and Fertilizers: Ammonium compounds, such as ammonium nitrate and ammonium sulfate, are commonly used in fertilizers. They provide essential nitrogen to plants, which is crucial for their growth and development. 2. Water Treatment: Ammonium compounds are used in water treatment processes to remove contaminants such as heavy metals and harmful ions. These compounds are exchanged with ions in water, allowing the removal of undesirable substances. 3. pH Control: Ammonium salts can act as buffers to regulate the pH of solutions in various applications, including chemical laboratories, industrial processes, and personal care products. 4. Food Industry: Ammonium compounds are used in the food industry as leavening agents (a substance used in dough to make it rise) in baking. Ammonium bicarbonate is an example of a compound that is used in baking. 5. Pharmaceuticals: Ammonium compounds are used in pharmaceutical production, where they can be used as active ingredients or as part of formulations. 6. Textile Industry: Ammonium compounds are employed in dyeing and finishing textiles. They can be used to modify the properties of fabrics and improve dye uptake. 7. Analytical Chemistry: Ammonium compounds are often used in analytical chemistry techniques, such as ion exchange chromatography and mass spectrometry, to separate and identify various substances. 8. Fire Retardants: Ammonium compounds can be used in fire-retardant materials, which helps to reduce the flammability of certain products like plastics, textiles, and wood. 9. Cosmetics and Personal Care: Ammonium compounds are found in various cosmetic and personal care products, including shampoos, conditioners, and skin creams, which can act as stabilizers or pH adjusters. 10. Electroplating: Ammonium salts are used to deposit metal coatings on the surfaces in electroplating processes. They facilitate the electrochemical reactions required for plating. 11. Phosphate Recovery: Ammonium compounds can be utilized in wastewater treatment to recover phosphates from sewage, which can then be used as a fertilizer. 12. Biotechnology: Ammonium salts are used in various biotechnological applications, such as in preparing cell culture media to grow microorganisms and cells. 13. Cleaning Products: Ammonium compounds are found in many cleaning products, such as disinfectants and household cleaners, due to their antimicrobial properties. Ammonium SaltsAmmonium salts are a class of chemical compounds that contain the ammonium cation (NH4+) combined with various anions. The ammonium cation is a positively charged polyatomic ion consisting of one nitrogen atom (N) and four hydrogen atoms (H). Ammonium salts are commonly formed through the reaction of ammonia (NH3) with acids. The ammonia molecule acts as a base by accepting a proton (H+) from an acid to form the ammonium cation. Ammonium salts are ionic compounds with the formula (R) 4 N+A-, where "R" represents hydrogen, alkyl, or aryl group and "A" represents an anion. When R is alkyl or aryl, the salts are known as quaternary ammonium salts. The quaternary ammonium cations are permanently charged, regardless of the solution's pH. Most ammonium salts are water-soluble. Cleaning agents, food additives, diuretics, surfactants, antiseptic agents, and disinfectants are all made from ammonium salts. They also have antimicrobial properties. Quaternary ammonium salts maintain cellular osmotic pressure. Quaternary ammonium compounds with long alkyl chains, such as benzalkonium chloride, cetylpyridinium chloride, dofanium chloride, benzethonium chloride, methyl benzethonium chloride, cetalkonium chloride, and didecyldimethylammonium chloride, have antibacterial, fungicidal, and antiviral activity. Since anionic detergents deactivate these quaternary ammonium compounds, they should not be used in hard water. They are commonly used as sanitizers. One of the most common applications of quaternary ammonium salts in organic synthesis is as phase transfer catalysts (PTCs), which catalyze the reaction between ionic and organic reactants. Chlormequat chloride acts as a plant growth regulator by inhibiting gibberellin production. When an amine is added to a strong acid solution, such as hydrochloric acid, the amine nitrogen atom is protonated to produce an ammonium salt. RNH2 + HCI ------à RNH3+Cl- Ammonium salts of low molecular weight are soluble in water if the amine's hydrocarbon portion is small because an ammonium salt's nitrogen atom has a positive charge, and ammonium salts are more water-soluble than amines. Drugs containing an amino group are often prepared as ammonium salts to improve their solubility in body fluids. The ammonium salts of many drugs are more stable and less prone to oxidation than the amine itself. Also, ammonium salts have higher melting points and virtually no odor. For example, ephedrine melts at 79 °C and has a fishy odor. Its hydrochloride salt, used in cold and allergy medications, melts at 217 °C and is odorless. Following are some examples of ammonium salts:
Detection of Ammonium Ion:1. Smell Test: Ammonium ions can be detected by the characteristic odor of their ammonia gas. To perform this test, add a few drops of sodium hydroxide (NaOH) or any other strong base, like Potassium hydroxide (KOH), Lithium hydroxide (LiOH), etc., to a solution containing ammonium ion. Heat this solution. The ammonium ions in the solution will react with the base to produce ammonia gas that can be detected from its choking odor. 2. Red Litmus Paper or Universal Indicator Paper: When a piece of red litmus paper is placed near the opening of the container containing the solution, it turns the damp red litmus to blue, confirming the presence of ammonium ions. 
3. Formation of White Fumes: When a substance containing ammonium ions is treated with a strong base, such as sodium hydroxide (NaOH), white fumes of ammonia gas (NH3) may be observed. These fumes are formed due to the release of ammonia gas when ammonium ions react with hydroxide ions. 4. Formation of Colored Complexes: Some metal ions form colored complexes with ammonium ions. For example, when ammonium ions are added to a solution of copper sulfate (CuSO4), a deep blue complex called tetramminecopper (II) complex forms, indicating the presence of ammonium ions. 5. Solubility Test: Many ammonium salts are highly soluble in water. If a solid substance readily dissolves in water and the solution exhibits properties such as a change in pH or the release of an ammonia-like odor, it could suggest the presence of ammonium ions. 6. Nessler's Reagent Method: Nessler's reagent is a yellow-brown solution that forms a brownish-red color in the presence of ammonium ions. This method is relatively simple and is often used for qualitative detection. 7. Indophenol Method: Ammonium ions react with sodium hypochlorite and phenol to form a blue indophenol complex. The intensity of the blue color is proportional to the concentration of ammonium ions and can be measured using a spectrophotometer. 8. Ion-Selective Electrodes: Ion-selective electrodes (ISEs) can measure the concentration of specific ions in a solution. These ion-selective electrodes are designed specifically for ammonium ions. These electrodes have a membrane that selectively allows ammonium ions to pass through, generating an electric potential proportional to the ammonium ion concentration. 9. Titration: Ammonium ions can be titrated using a strong base, such as sodium hydroxide (NaOH). The endpoint of the titration is reached when all the ammonium ions have reacted with the base, resulting in a pH change that can be detected using a pH indicator. 10. Chromatography: Ion chromatography can separate and quantify various ions in a solution, including ammonium ions. This method relies on the differential migration of ions through a chromatographic column under the influence of an electric field. 11. Fluorescence Spectroscopy: Fluorescent dyes can detect ammonium ions based on their ability to bind to the ions and produce a fluorescent signal. The intensity of fluorescence can be correlated with the concentration of ammonium ions present. 12. Mass Spectrometry: Mass spectrometry can identify and quantify ions, including ammonium, based on their mass-to-charge ratios. This method is more advanced and often used in research and analytical laboratories. 13. Ammonia Gas Sensing: Gas sensors can be used to detect gaseous ammonia (NH3). These sensors often monitor ammonia levels in the environment or industrial settings. The choice of method depends on factors such as the sensitivity required, the sample matrix, the concentration range of ammonium ions, and the equipment available. It's important to note that different methods offer different sensitivity levels, accuracy, and ease of use.
Next TopicAmmonium-Dichromate
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share













