AmmonificationNitrogen is required for all living species to live. Many biomolecules, including proteins, DNA, and chlorophyll, rely on it for their formation. Even though Nitrogen gas is abundant in the atmosphere, most species are unable to access it in this form, making it a limiting resource that affects primary productivity in many ecosystems on a regular basis. Nitrogen is inaccessible to primary producers such as plants until dinitrogen gas is transformed to ammonia. 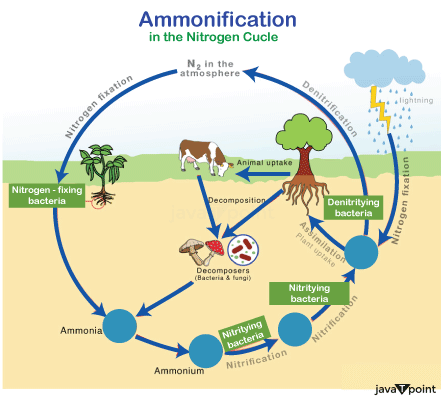
Nitrogen exists in inorganic and biological forms, as well as dinitrogen and ammonia. As a result, as organisms use nitrogen for development and, in some circumstances, energy, it undergoes a variety of distinctive changes across the environment. Nitrogen is primarily altered through nitrogen fixation, nitrification, denitrification, ammonium generation, and anammox. The biosphere's production is mostly based on the oxidation of nitrogen into its many oxidation states, which is achieved by a diverse range of microorganisms. AmmonificationThe ammonification process, which provides organisms with the nitrogen they need to survive, is part of the nitrogen cycle. Ammonification is the process by which microscopic creatures such as bacteria or other decaying critters convert nitrogen-containing compounds from dead organic matter into simple molecules such as ammonia. These simpler materials are beneficial to the environment. What Happens to Bacteria During the Ammonification Process?Nitrogen (organic form) is released from a living thing's cells or tissues in the form of amino acids and DNA when it dies. Fungi, prokaryotes, and other microorganisms also work together to break down the tissue and convert organic nitrogen to inorganic nitrogen. All microbes utilise this inorganic form. Steps in the Ammonification Process
Effects of Ammonification
Examples of AmmonificationBacillus, Proteus, Clostridium, Pseudomonas, and Streptomyces are examples of ammonifying microorganisms. NitrificationNitrification, which converts ammonia to nitrite and, eventually, nitrate, is another critical stage in the global nitrogen cycle. The great majority of nitrification is accomplished aerobically and only by prokaryotes. Different species of microorganisms perform the two primary steps of nitrification. The first stage involves ammonia-oxidizing bacteria converting ammonia to nitrite. To convert ammonia to nitrite via the intermediary hydroxylamine, aerobic ammonia oxidizers require two different enzymes: ammonia monooxygenase and hydroxylamine oxidoreductase. Nitrosofiers are notorious for their extremely slow growth since the process offers relatively little energy in comparison to many other types of metabolism. Aerobic ammonia oxidizers are autotrophs that use ammonia as their energy source rather than light to repair carbon dioxide and create organic carbon. Nitrogen fixing by a diverse spectrum of microorganisms is more common among prokaryotes than ammonia oxidation. Previously, it was thought that only a few bacterial species were responsible for all ammonia oxidations. We now have a limited understanding of the physiological diversity of ammonia-oxidizing archaeons because just one of them has been grown in pure culture. The second stage of nitrification is the conversion of nitrite to nitrate. This is done by a subset of prokaryotes known as nitrite-oxidizing bacteria. Growth yields are exceedingly low because nitrite to nitrate oxidation, like ammonia oxidizers, produces very little energy. In reality, a huge number of ammonia or nitrite molecules must be oxidised before an ammonia or nitrite oxidizer can fix one carbon dioxide molecule. Nitrite and ammonia oxidizers are frequent in aerobic settings. They've been thoroughly studied in a number of natural settings, including soils, estuaries, lakes, and open oceans. Ammonia- and nitrite-oxidizers, on the other hand, play a vital function in wastewater treatment plants by removing potentially dangerous levels of ammonium that could contaminate receiving waters.
Next TopicBlood Clot
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share









