Bacterial MorphologyThe morphology of bacteria is incredibly varied. Adaptive forces that optimize bacterial fitness result in certain morphologies. Important biological processes like as nutrition uptake, motility, dispersion, stress tolerance, and interactions with other organisms are all influenced by shape. Although bacterial species' distinctive form stays constant over many generations, periodic alterations occur during the cell division and life cycles, and external factors may alter these variations. The net-like peptidoglycan (PG) sacculus is ultimately responsible for bacterial shape. Multiple variables contribute to the species-specific form of the PG sacculus at any point in the cell cycle. Some morphological determinants operate as a cytoskeleton, guiding biosynthetic complexes in space and time, while others change the PG sacculus after biosynthesis. A growing body of data suggests that morphogenetic mechanisms play a significant role in bacterial-host interactions, including pathogenicity. 
People have, in fact, overlooked the diversity of bacteria in terms of their structure. For example, star-shaped, moustache, serpentine, and branched bacteria also exist, along with the well-known cocci, spiral, and rod-shaped bacteria. These new bacterial morphologies are indeed undetermined. The bacterial species' typical shape is retained over many generations, although it is regularly changed within specific limitations throughout the bacterial division and life cycles. Bacterial form is regulated by genetics, but physical forces (both internal and external) placed on cells are becoming more widely acknowledged as important factors in morphogenesis. Despite these influences, shaping maintenance must be an active process led by sophisticated regulatory circuits to sustain stable bacterial morphology throughout generations. The emergence of mutated bacteria has shown this with abnormal morphology due to different mutations. The bacterial shape offers more than just a shape to the bacteria. It helps the bacteria in the development of multicellular aggregates, during colonization in the Eukaryotic host, during predation, in motility and resistance. As a result, morphogenesis should be regarded as an important evolutionary and adaptive mechanism that considerably contributes to prokaryotic ubiquity and diversity. The primary determinant of the bacterial shape, which acts as the main ingredient in forming a distinct bacterial morphology, is peptidoglycan called sacculus. It is a macromolecular structure, polymeric in nature, that is known to surround the cytoplasmic membrane. It has been proved to act as the only solid feature in the bacterial envelope hence determining its unique shape. This form of peptidoglycan is found in almost all the bacteria as a surrounding membrane of the cytoplasm, just like an elastic net. Although the exceptions are also there, the peptidoglycan seculars are basically a polymer of glycan change that crosses together. N-acetyl glucosaminyl-N-acetyl-muramyl-L-alanyl-D-glutaminyl-L-meso-diaminopimelyl-D-alanyl-D-alanine is the monomer of this net-like peptidoglycan. The difference between structures formed from the same monomeric unit comes as a result of differences in accessory processes that might affect the basic subunit, or there might be a change in the structure of the Amino acid sequence of the stem peptide. The precursor for the biosynthesis of this peptidoglycan is produced by the enzyme MurA-F. The precursors are uridine diphosphate N acetylglucosamine and UDP murNAC pentapeptide found in the cytoplasm. 
The membrane-anchored lipid I is produced by the enzyme MraY, which combines the UDP-MurNAc-pentapeptide with undecaprenyl phosphate. Murg then adds GlcNAc to lipid I, resulting in inwardly orientated lipid II molecules. Flippase MurJ carries out the translocation to the outer face of the cytoplasmic membrane along with Amit. In the process, the other proteins which are known to play a role are SEDS (shape, elongation, division, and sporulation) proteins RodA and FtsW. The GlcNAc-MurNAc-pentapeptide moiety of lipid II becomes available to enzymes with glycosyltransferase (GT) and transpeptidase (TP) activity, which catalyze linear polymerization and peptide cross-linking, respectively, after it is transported to the exterior side of the cytoplasmic membrane. The undecaprenyl diphosphate released during the polymerization step is flipped back, dephosphorylated, and employed to transport new precursors in a cyclical manner. Bifunctional proteins having GT and TP functions are ubiquitous, and monofunctional representations of both activities exist concurrently. SEDS protein RodA was recently discovered in Bacillus subtitle as a new GT enzyme, and it has shown to play a similar role in E coli. The common type of enzyme that is involved in the cross-linking of polymer is DD transpeptidase. This enzyme is inhibited by covalent beta lactame binding, and it was discovered as a penicillin-binding protein (PBP). Peptidoglycan show DD cross-links the third position of diamino acid in one subunit stem peptide and the fourth position of D alanine. The cross-link could be direct or via an intermediate peptide. It is rather prevalent in the prokaryotic system, where crossing-linking methods involve particular enzymes and stereochemistry. Adding any additional material will result in a simultaneous breakdown of the pre-existing bonds in the peptidoglycan layer because it is already a covalently closed structure, and peptidoglycan hydrolysis will break the bonds to allow sacculus growth. 
People are showing more interest in the field of studying modifications that cause shape alterations that result in the formation of new structures throughout the cell and life cycle of bacteria. PG hydrolases play a major role in PG remodeling and maturation. These enzymes attack every glycosidic and peptidic bond in the PG fabric as a whole. Many hydrolases may be encoded by organisms, many of which are redundant (E. coli has 35). Aside from the growth process, the sacculus has a complicated and dynamic metabolism involving a significant number of proteins that aren't directly engaged in precursor integration. Subunit ageing, growth status, dietary circumstances, population density, and stress response have all been linked to changes. This article also explains how sacculi are shaped and transformed in Gram-negative bacteria to generate typical bacterial morphologies. The proteins involved, as well as the processes that underpin them, will be discussed. Cell Shape GenerationThe PG sacculus keeps a fixed form due to its covalently closed, net-like structure, which it imposes on the bacterial cell body. Due to the elastic nature of the peptidoglycan fiber, sacculi are able to preserve the definite shape of the cell even if there is some deformability. The sacculi, on the other hand, lack the information and/or traits necessary to define their particular shape. It has been suggested that epigenetic structural information be encoded in the 3D arrangement of the molecule. Even after so much of studies, there is still no solid evidence to prove this theory of peptidoglycan responsible for the shape of bacteria. Also, the recent understanding of the seculars is somewhat confusing to some people. Even if the older material's pre-existing order (or lack thereof) favors a specific disposition of arriving new antecedents, this does not always imply a universal shape-defining function. As a result of this understanding, people have come to a conclusion that though sacculus is responsible for keeping a definite size and form of the bacteria, the production of that shape is still dependent on some dynamic and topology of other biosynthetic complexes and not alone secular itself. The simple expansion of a closed net under cytoplasmic turgor pressure imposes several essential limits on the inclusion of new material necessary for successful enlargement. Only thickening would ensue from the simple attachment of arriving precursors to the sacculus. Morphogenetic methods must be used to circumvent these limits. A new form of peptidoglycan endopeptidases has been found in E coli, bacillus subtitles, and vibrio cholerae, which allow the growth of bacteria by cleaning the existing crossing peptide. 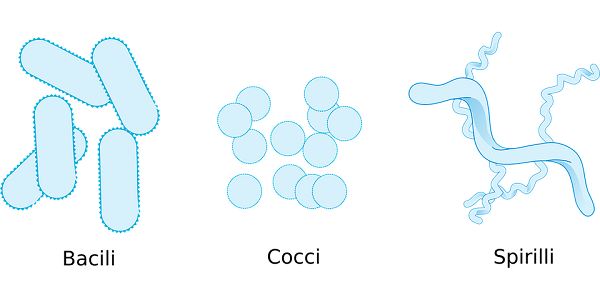
When talking about bacteria, the process of morphogenesis requires not only the periodic divisions but also the physical expansion event to increase the number of individuals. However, if the insertion of new material and the cleavage of existing cross-links occurred continuously and equally throughout the whole surface of the sacculus, the developing structure would expand uniformly. This system would not be able to differentiate new characteristics on its own. Incorporation must occur at varying rates in different regions and for set periods of time to form shapes other than a sphere. For example, budding would need a higher rate of precursor integration at the budding location than elsewhere. Symmetrical binary fission is the most well-known division process in model organisms, and it provides an easy, straightforward technique for ensuring shape retention. Alternative division methods do, however, exist. The very criteria for the bacterial division are that both the biochemical components and genetic material, which are important for genetic potential, should be distributed equally. The division must be managed in such a manner that no additional divisions are permitted until the daughter cells have met these requirements. The process of cell division in the bacterial species is different for different bacteria. It results in the formation of child cells that differ in terms of physiology, shape, and size from the mother cells. In such cases, these child or juvenile cells have to go through a lengthy process of developmental program in order to form a defined desired morphology before they go into the next cycle of division. The production of two cells is through scission of the bacterial cell wall. The place at which this cleavage would occur is definite at a definite phase of the cell cycle which maintains the integrity of the cell, and this process is called cytokinesis. During cytokinesis and growth, that is, enlargement and differentiation of the bacterial cell, a shared substrate is sacculus which is mediated by the complex enzymes. Thanks to recent developments in genetics and imaging tools, the factors responsible for the dynamics and structure of PG biosynthetic complexes are progressively being unraveled, as explained below. Peptidoglycan Synthesis Positioning and Guidance: Cytoskeletal ElementsSince PG determines the shape of bacterial cells, controlling the location and timing of PG production and breakdown during the cell cycle is critical. In massive, tightly controlled protein complexes, bacteria employ cytoskeletal components to arrange proteins involved in PG production and hydrolysis. Morphological Determinants Not Dependent on PeptidoglycansThere are PG-independent variables as well as morphological determinants that alter the PG sacculus. The extra twisting of the bacteria or the spiral or flat waveform of bacteria is due to the presence of the periplasmic flagellum. The reason periplasmic flagella can change their form its shape is because the sacculus is deformed by it, and in turn, the sacculus deforms the flagella resulting in a cell shape. Even in a very strict environment, this dynamic mechanism enables the bacteria to move or migrate from one place to another. Some bacteria like Borrelia burgdorferi's depend on their motility for pathogenicity. Membrane composition is another PG-independent morphological factor, as established for the rod-shaped Rhodobacter sphaeroides. R. sphaeroides is virtually spherical when the membrane lipid cardiolipin is decreased. The mechanism by which a reduction in cardiolipins leads to altered cell shape in R. sphaeroides is unknown. Due to their shape, cardiolipin molecules are preferentially localized in regions with greater membrane curvature, such as cell poles and cell division sites. It is assumed that the spherical cells have more cardiolipin because spherical cells have more proportion of their membrane in a curved form than the rod-shaped cells. Cardiolipin is abundant in E. coli minicells, which have a large proportion of their membrane in a highly curled form.In conclusion, it can be said that the composition of the membrane directly or indirectly influences the shape of the cell. 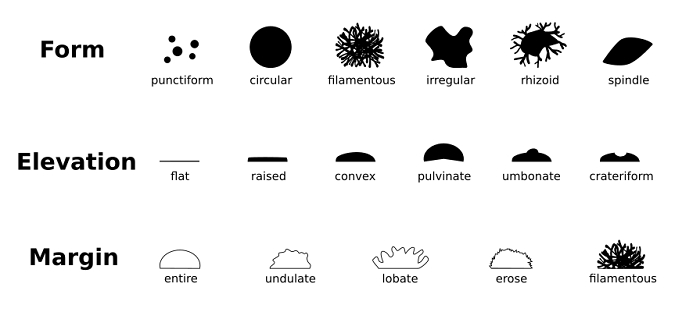
A Bacterial Cell's Shape Changes Throughout Its LifeDuring the cell cycle, several bacterial species experience drastic morphological changes (dimorphic or polymorphic bacteria). The morphology of sacculus can be changed by remodelling of spatiotemporal activation pattern of peptidoglycan biosynthetic complexes or through the changes in the frequency of cell division relative to the growth rate. Shape modification, on the other hand, often involves "de novo" differentiation of cell sections or appendages, such as "points" in Stella vacuolate or prostheca in Asticcaulis biprosthecum and Hyphomonas neptunium. Additional factors are required in these scenarios to determine when and where new complexes are formed and activated. The first morphogenetic elements with such powers have been found in C. crescentus and similar species. If "adequate and essential" modular components are required for these sorts of form alterations, such elements might be good instruments for manipulating shape in biotechnologically important species. Changes in Morphology During the Cell CycleThe best model organism studied to study the changes in the morphology during the cell cycle is C. crescentus, an alphaproteobacterium with a cell cycle-dependent morphology. A stalk arises from the previously flagellated cell pole throughout development, giving juvenile flagellated swarmer cells a curved rod form. The cell finally splits asymmetrically: the stalked mother cell may divide again right away, but the daughter cell must first evolve into a stalked cell before undergoing another round of division. The process of cell division happens exclusively in stalk cells. This is dependent on the coordination between Z ring construction and chromosomal segregation in a special temporal manner through the activities of MipZ, CtrA, and DnaA, among other proteins. Although the transcriptional regulators TacA and StaR are important in stalk development, tacA and staR mutants still generate stalks when phosphate is depleted, suggesting the presence of additional stalk regulators. Even though many factors which are involved in the elongasome complex have been established, for example, bactofilin and pbpc, localized at the base of the stock and elongasome component rod a and mreb. Still, the efficient method by which the stretching of stock happens is unknown. 
Asticcacaulis species are related to C. crescentus and, like C. crescentus, produce stalks throughout the cell cycle. Distinct Asticcaulis species have different stalk locations: A. excentricus has one subpolar stalk, while A. biprosthecum has two bilateral stalks near the midcell. Another alphaproteobacterium, Hyphomonas neptunium, has a budding bacterium with a cell cycle-dependent shape. A stalk that develops from the mother cell produces new progeny. This cell division is asymmetric, much as in C. crescentus: the ovococcoid daughter cell can only divide after evolving into a stalked cell. These bacteria's cell cycle-dependent morphology is due to PG incorporation at certain cellular sites depending on the cell cycle stage. Buds also emerge from the stalk via the remodeling of the stalk's tip. Further study is required to fully comprehend the processes that control and construct this morphogenetic program. Studies of di- or polymorphic bacteria have offered a greater knowledge of the control and coordination of morphogenesis, as the examples above demonstrate. Since just a few bacteria with cell cycle-dependent morphologies have been studied, future research on this subject is anticipated to uncover many additional regulatory networks. 
Environmental Changes Influence Morphological ChangesChanges in environmental factors have a significant impact on bacteria. Under specific circumstances, morphological changes occur in a variety of animals. These alterations might be connected to a metabolically inactive condition, a requirement to boost nutritional intake, or a need to avoid predators. The bacteria can also enter a condition called viable but not culturable condition, which is the dormant phase in the bacterial life cycle. Low-temperature exposure or any restrictions on the nutrients might lead to this phase. In several species, the formation of VBNC forms is linked to morphological alterations. Many Gram-negative pathogens may switch between the rod and coccoid forms. In some cases, these morphological changes have been linked to the regulation of cell envelope/wall genes. The bacterial growth stage can also influence bacterial morphology and PG topology. In the stationary phase, E. coli's stringent response controls the downregulation of PG synthesis, and the sacculus experiences a variety of structural modifications, including greater cross-linking (including LD-crosslinks) and shorter chain length. The same dynamics apply to V. cholerae, but it additionally exhibits RpoS-dependent cell wall chemical editing mediated by non-canonical D-amino acids. During the stationary phase, C. crescentus develops a morphological adaptation that causes the cells to lengthen, narrow, and become helical. Another morphological adaptation is a significant extension of the stalk by C. crescentus (and related such as Asticcaulis species) in response to phosphate constraint. This extension of the stalk seems to be a strategy for either increasing phosphate absorption capacity or elevating the cell body above the surface. The processes behind these adaptations in C. crescentus are unknown. 
Multiple bacteria expand dramatically in length in response to environmental challenges via a mechanism known as filamentation, which involves stopping cell division while sustaining cell growth. In E. coli, the SOS response induces the division inhibitor Sula, which causes cell filamentation. During urinary tract infections, the cell division gene damX is required for filamentation in uropathogenic E. coli (UPEC). In addition to filamentation, UPEC undergoes additional morphological changes as it forms non-motile, rod-shaped intracellular bacterial communities (IBCs). These begin as coccoids that invade the cytoplasm of bladder umbrella cells and then evolve into slower-growing coccoids that form more organized biofilm-like communities (mid-IBCs). Within these mid-IBCs, a tiny minority of cells continue to develop into filaments. The coccoid UPEC cells eventually become motile and bacillary (late IBCs), lysing the host cell and releasing filaments and motile rods for further invasion rounds into nearby bladder cells (egress and second-generation IBCs). Since each IBC represents a single invasion event, the morphological alterations seen within these communities are most likely part of a developmental program. Each morphotype is thought to aid intracellular growth and future infection cycles.
Next TopicChemosynthetic Bacteria
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










