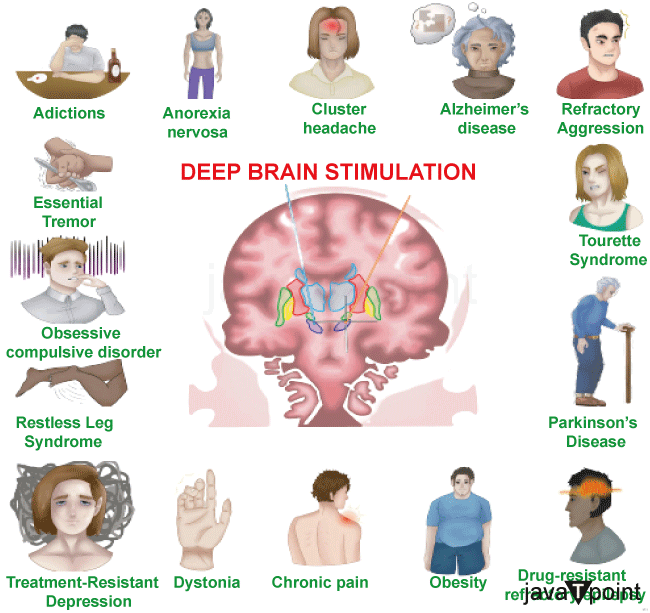
Deep Brain StimulationDeep brain stimulation (DBS) is a neurosurgical procedure involving implanting a neurostimulator device that sends electrical impulses to specific targets in the brain (the brain nucleus) through implanted electrodes. It treats movement disorders like Parkinson's disease, essential tremor, dystonia, and other conditions like obsessive-compulsive disorder (OCD) and epilepsy. DBS directs regulated alterations in brain activity even if its fundamental concepts and processes are not completely understood. Since 1997, DBS has been approved by the Food and Drug Administration as a therapy for Parkinson's disease (PD) and essential tremor. Obsessive-compulsive disorder (OCD) was licensed for DBS in 2009, and epilepsy was allowed for DBS in 2018. In clinical studies, DBS has been investigated as a possible therapy for persistent pain in various affective illnesses, including severe depression. The method is one of the few in neurosurgery that permits blinded research. 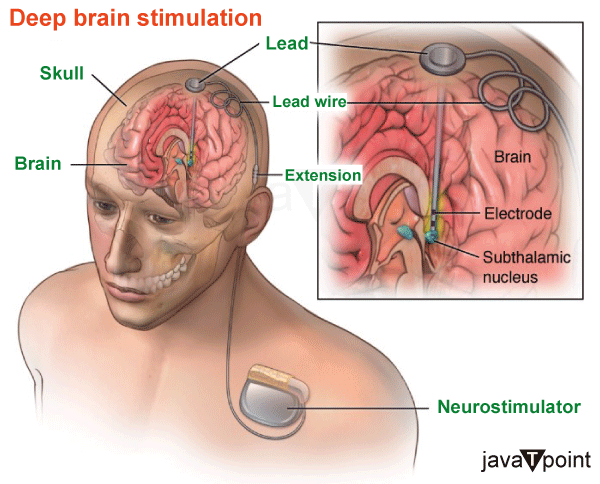
Three components of a DBS system are inserted within the body: Neurostimulator - A programmable pacemaker with a battery that produces electric pulses. It may be positioned in the belly or under the skin of the chest just below the collarbone. Lead - An electrode-equipped coated wire that sends electrical pulses to the brain tissue. It is inserted within the brain and attached to an extension cable through a tiny skull hole. Extension - A lead-to-neurostimulator connection made using an insulated wire. It is positioned under the skin and extends from the scalp to the chest through the area behind the ear. The patient turns the DBS system on and off using a portable device. Utilizing a wireless device, the doctor configures the stimulator parameters. As the state of a patient's health varies over time, the stimulation settings may be changed. Unlike other procedures like thalamotomy and pallidotomy, DBS does not harm the brain's tissue. Therefore, the DBS technique may be undone if better therapies emerge in the future. Dyskinesias, the uncontrollable wriggling motions brought on by large dosages of levodopa medication, may be greatly reduced by DBS. Typically, DBS will assist in lessening the severity of your symptoms so that smaller medicine dosages may be utilized. Parts of the basal ganglia are either under or over-stimulated in Parkinson's disease. Tremor, rigidity, and stiffness take the place of normal mobility. By changing the faulty electrical pathways and assisting in stabilizing the feedback loops, the DBS of certain ganglia reduces symptoms. The following parts of the brain may be electrodes: Subthalamic Nucleus (STN)- Beneficial for dyskinesia, dystonia, dystonic tremor, and stiffness. Parkinson's disease treatment is most often utilized. Thalamus (VIM) - Helpful for tremors. Treatment of essential tremors often involves it. Globus pallidus (GPi) - Beneficial for dyskinesia, dystonia, dystonic tremor, and stiffness. Parkinson's disease and dystonia are both treated with it. DBS is employed When drugs can no longer maintain a patient's high standard of living. How does Deep Brain Stimulation Work?The parts of the brain that govern movement are affected by disordered electrical impulses, which contribute to the movement-related symptoms of Parkinson's disease and other neurological diseases. DBS can stop the erratic impulses that result in tremors and other movement-related problems when it is effective. Neurosurgeons insert one or more cables, called "leads," into the brain after tests pinpointing the ideal location. The leads are attached to a tiny neurostimulator (electric generator) placed under the patient's collarbone, much like a cardiac pacemaker. The neurostimulator delivers a steady stream of electric current pulses through the leads and into the brain. The neurostimulator is programmed to provide an electrical signal a few weeks after it is implanted. This programming procedure could need many visits over weeks or months to ensure the current is appropriately adjusted and producing good outcomes. The doctor's device adjustments aim to achieve the best possible symptom management while minimizing adverse effects. Who is the Candidate for Deep Brain Stimulation?DBS involves more than simply surgery. People interested in receiving treatment with DBS should be ready to devote time to the process since it entails several assessments, procedures, and consultations before and after the surgery. For instance, those who don't live near a hospital that performs DBS surgery could have a long commute to their appointments. Depending on the patient's insurance coverage, the operation, pre-operative assessment, and post-operative follow-up may be costly. The FDA has authorized DBS surgery as a therapy for Parkinson's disease, and Medicare and the majority of private insurers will pay for the operation; however, the level of coverage will vary depending on the unique policy of each patient. Prospective patients should anticipate reasonable outcomes with DBS. Although DBS may significantly improve the quality of life and movement symptoms associated with Parkinson's disease in carefully chosen individuals, it is unlikely to restore anybody to full health. Deep Brain Stimulation Surgeries and ImplantationDBS entails two procedures separated by three to six weeks to provide the patient with enough recovery time. Throughout your stay, you will get top-notch medical care from a group of doctors, including a neurosurgeon, an electrophysiologist, and an anesthesiologist. First stage - Electrodes are inserted into certain brain parts during this procedure. Our specialists will use an MRI (magnetic resonance imaging) scan to map the brain and pinpoint the locations of the electrodes. The whole process will be done while you are awake. This will enable you to reply to the neurologist and other medical personnel as they conduct certain tests to ensure the electrodes are positioned in the best possible places. The University of Michigan is also one of the few hospitals in the nation where a speech therapist will be on hand to watch you while the treatment is being done. Speech issues are one of the recognized negative effects of DBS. Because of constant monitoring, U-M enjoys having a low incidence of speech adverse effects among patients. Most people need to spend the night in the hospital following the surgery. Second stage - The pulse generator, also known as a "brain pacemaker," will be implanted at this stage and coupled to the electrodes positioned during stage one in your chest region, close to the collarbone. For the process, you will be asleep throughout. The lead will be attached, and your doctor will implant the generator. The procedure is often outpatient and is fairly well tolerated. DBS has a lot to offer. A neurologist may configure the generator to be specific to each patient's needs. Additionally, the process is reversible. Symptoms significantly improve for the majority of people. But there are hazards, just as with any brain surgery. The stroke risk with DBS is one in one hundred, and the infection risk is one in fifty. More people might benefit from DBS today than are already benefited by the treatment. Only 7% of Parkinson's disease patients and 1% of tremor sufferers in Michigan who might benefit from the operation have had DBS, according to statistics. Medical Uses of Deep Brain Stimulation1. Parkinson's DiseaseDBS treats certain Parkinson's disease symptoms that are ineffectively managed by therapy. High-frequency (> 100 Hz) stimulation is used to treat Parkinson's disease (PD) by simulating the clinical symptoms of lesioning in three target structures: the subthalamic nucleus (STN), internal pallidum, and ventrolateral thalamus. As long as they do not have serious neuropsychiatric issues, it is advised for persons with Parkinson's disease (PD) who have motor fluctuations and tremors that medication cannot properly manage or for those who are drug intolerant. Neural stimulators have been used to treat four brain regions in PD. The thalamus, globus pallidus internus, subthalamic nucleus, and pedunculopontine nucleus are among them. However, in everyday practice, the Subthalamic nucleus or the Globus Pallidus Internus is the focus of most DBS procedures. Dyskinesias uncontrolled shaking motions, are lessened by DBS of the globus pallidus internus. This makes it possible for the patient to take the right dosages of their drugs, notably levodopa, which improves symptom management. Direct brain stimulation of the subthalamic nucleus decreases Parkinson's symptoms. This makes it possible to lower the dosage of anti-parkinsonian drugs. While DBS of the thalamus may aid with tremors, DBS of the PPN may help with gait freezing. These objectives are only sometimes used. The procedure of choosing the ideal DBS target is challenging. The most problematic symptoms, the dosage of levodopa the patient is presently taking, the effects and side effects of the existing drugs, and concurrent issues are only a few of the clinical factors utilized to choose the goal. In individuals with unmanaged depression, for instance, subthalamic nucleus DBS is not recommended since it may make their depression worse. DBS is often linked to a 30-60% improvement in assessments of motor scores. However, DBS is continually delivered with set settings and only partially controls the motor fluctuations that are a hallmark of Parkinson's disease. As a result, the idea of adaptive deep brain stimulation (DBS), a kind of DBS that automatically changes stimulation settings to Parkinsonian symptoms, was developed in recent years. ADBS devices are now being researched for use in therapeutic settings. 2. Tourette SyndromeAdults with severe Tourette syndrome who don't respond to standard therapy have been treated experimentally with DBS. Despite its early achievements, which have been extensively reported, DBS is still a highly experimental treatment for Tourette's, and additional research is required to establish if its advantages exceed its drawbacks in the long run. The technique is largely tolerated, but problems might arise due to "short battery life, abrupt symptom worsening upon cessation of stimulation, hypomanic or manic conversion, and the significant time and effort involved in optimizing stimulation parameters." The operation is costly, intrusive, and requires long-term professional care. Given the less significant effects of this procedure shown in the Netherlands, benefits for severe Tourette's remain uncertain. Tourette's is more prevalent in pediatric populations and often goes away in maturity; thus, using this therapy on kids is not generally advised. Because the diagnosis of Tourette's is dependent on a history of symptoms rather than an evaluation of brain activity, it may not always be clear how to use DBS for a specific individual. The Tourette Association of America assembled a team of professionals to provide guidelines governing the usage and possible clinical trials of DBS for TS due to concerns over its use in TS therapy. According to Robertson, by 2011, DBS had been applied to 55 individuals. At the time, it was still considered an experimental therapy, and he advised that it "should only be performed by experienced functional neurosurgeons operating in centers that also have a dedicated Tourette syndrome clinic." Malone et al. (2006) said, "only patients with severe, debilitating, and treatment-refractory illness should be considered, while those with severe personality disorders and substance-abuse problems should be excluded." As an invasive therapy, DBS is presently only advised for seriously afflicted, treatment-refractory TS adults, according to Du et al. (2010). Singer (2011) advises a cautious approach "pending determination of patient selection criteria and the results of carefully controlled clinical trials." Viswanathan et al. (2012) suggest that those with "a severe functional impairment that cannot be managed medically" should consider DBS. 3. EpilepsyUp to 36.3% of people living with epilepsy have medication resistance. These patients have high mortality and morbidity risks. Neurostimulation techniques, including DBS, vagus nerve stimulation, and responsive neurostimulation, may be used when surgery is not an option. For the treatment of epilepsy, targets besides the anterior nucleus of the thalamus, such as the centromedian nucleus of the thalamus, the cerebellum, and others, have been researched. Adverse Effects of Deep Brain StimulationDBS entails the dangers of major surgery, with the surgical team's expertise impacting the complication rate. Infection (3-5%) and bleeding (1-2%) are the two main side effects. Following DBS, there is a risk of neuropsychiatric side effects such as euphoria, apathy, hallucinations, hypersexuality, cognitive impairment, and depression. These adverse effects, however, may be short-lived and connected to:
During surgery, the brain might move somewhat, which causes the electrodes to move or become loose from their original position. Although electrode misplacement is generally simple to detect with a CT scan, it may result in more serious issues, including personality changes. Additionally, postoperative problems, including brain hemorrhage, are possible. Swelling of the brain tissue, moderate confusion, and tiredness are common side effects after surgery. A follow-up appointment is performed to remove the sutures, activate the neurostimulator, and program it after 2-4 weeks. A surprisingly high danger of the surgery was impaired swimming abilities; after deep brain stimulation, numerous Parkinson's disease patients lost their swimming ability.
Next TopicHuman Brain Weight in Kg
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share









