Passive TransportPassive transport is a type of transport in which a substance moves from its higher concentration to its low concentration. This type of movement of molecules across a membrane occurs without using energy, so it is termed passive transport. On the other hand, when energy (ATP) is needed, the movement is called active transport. Active transport moves molecules against their concentration gradient, from the low concentration to the high concentration. 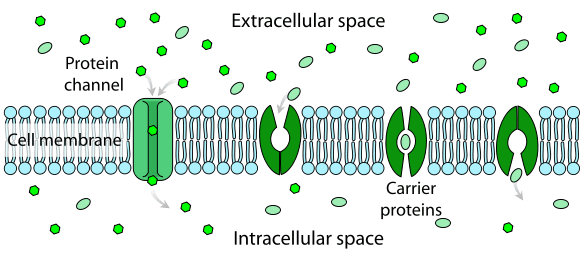
Different types of Passive TransportAs substances move across biological membranes, passive transport may or may not require the assistance of membrane proteins. There are 4 types of passive transport:
1. Simple Diffusion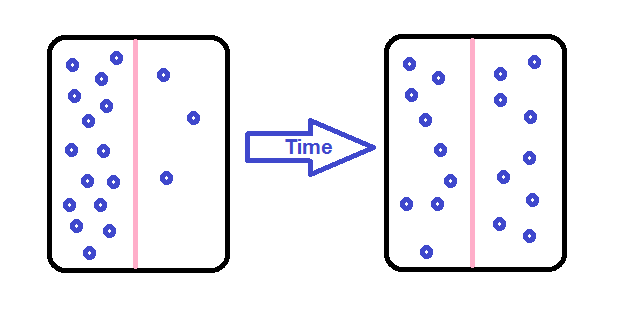
Let's take an example of Simple diffusion with some real-life scenarios- Have you recently passed through airport security? If so, then you may have noticed that it has been carefully designed to allow some objects to pass through (e.g. passengers with tickets) and others (e.g. weapons, explosives, and bottled water). Flight attendants, captains and airport staff move quickly through dedicated channels, while regular passengers go through slower, sometimes long queues. In many ways, airport security is very similar to the plasma membrane of a cell. Cell membranes are selectively permeable, regulating which substances can pass through and how much of each substance can enter or exit at a given time. Selective permeability is essential to cells` ability to obtain nutrients, eliminate wastes, and maintain a stable interior environment different from the surroundings (maintain homeostasis). The simplest forms of transport across a membrane are passive transport. Passive transport doesn't need the cell to get through any energy and involves a substance diffusing down its concentration gradient across a membrane. A concentration gradient is just a space over which the concentration of a substance changes and substances will naturally move down their gradients, from an area of higher to an area of lower concentration. In cells, some molecules can move down their concentration gradients by crossing the lipid portion of the membrane directly, while others must pass through membrane proteins in a process called facilitated diffusion. Here, we`ll look at some examples of Simple diffusion. Examples of simple diffusioni) Movement of oxygen and carbon dioxide One of the best examples of simple diffusion is the movement of gases across animal membranes. For example, the movement of oxygen & carbon dioxide dissolved in the blood. The concentration gradient of these gases in the cell determines the direction of gas movement. In the alveoli, the oxygen concentration is higher than in the blood vessels when inhaled. Thus, the movement of oxygen occurs from the alveoli to the blood. In the same way, during exhalation, the concentration of carbon dioxide in the blood is higher than in the alveoli, which causes carbon dioxide to move into the lungs. A similar process occurs during the gas exchange between blood and cells. ii) In the Waste material In animals, waste is removed by simple diffusion. Urea, a waste product from the liver, is excreted into the bloodstream through a simple diffusion process. Similarly, waste products and toxins in the kidneys are removed through simple diffusion and water is absorbed. In some parts of the kidneys, separate active transport also occurs. iii) Nutrition in bacteria Prokaryotes like bacteria do not have a specialized mechanism for the movement of nutrition, water, gases and other solutes all the way through their body. They depend on simple diffusion for the movement of molecules within the cytoplasm. Further, the excretion of waste materials is also aided by simple diffusion as it occurs through the general body surface in bacteria. Applications of simple diffusionThe simple diffusion concept is applied in numerous fields, for instance, environment, food, medicine and more.
2. Facilitated TransportIn facilitated transport, which is also referred to as facilitated diffusion, molecules that are too big or polar to diffuse across the plasma membrane through simple diffusion need extra help to move in or out of the cell membrane. Further, transport proteins present in the plasma membrane allow these ions or molecules to move across the cell membrane. 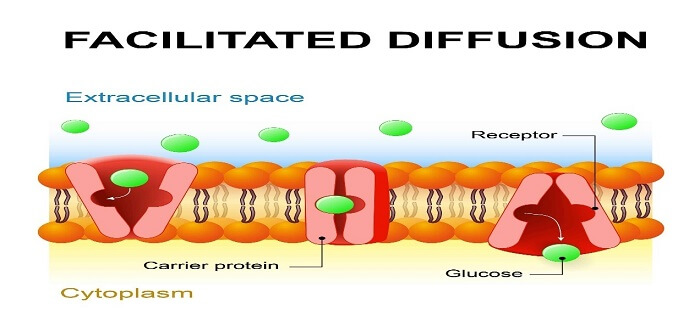
In the process, molecules need some extra help to get pass through the cell membrane and for that extra help is provided by the transport proteins present in the membrane. Moreover, molecules naturally move from a region of high concentration to a region of low concentration. Types of Membrane Proteins
Channel proteins allow molecules to pass through both directions; on the other hand, carrier proteins tie those specific molecules and let them in and out of the cell. Examples of facilitated diffusioni) Diffusion in Amino acid and Glucose Transport The transport of amino acids and glucose from the blood into the cell occurs through facilitated diffusion. As such, the small intestine is the main site for glucose and amino acid absorption into the blood and these solutes move through three processes; facilitated diffusion, simple diffusion and active transport. Moreover, larger molecules like amino acid and glucose need carrier proteins also referred to as glucose transporters or amino acid permeases, which carry blood in the cell. ii) Gas Transport In blood, hemoprotein is the carrier macromolecule, whereas, in muscles, the carrier macromolecule is myoglobin. The diffusion of blood occurs due to higher pressure on one side of the membrane and a lower pressure one on the opposite side. A similar mechanism is concerned with the transport of carbonic acid gas and monoxide. 3. FiltrationFiltration is a biological, chemical and physical operation. Further, it is a process for separating solids from liquids and gases. Moreover, the selective absorption of nutrients within the body is also called filtration. The process doesn't need any energy and takes place on the concentration gradient. One of the prime examples of the biological filter is the kidneys. Capillary filtered the blood and while absorbing the vital molecules. The cell membrane permits solely those substances which are soluble and could easily pass through its pores in the method of filtration. One such real-life example of filtration is the making of coffee. In the process of making coffee, we can assume coffee filters as the cell membrane and can assume coffee grounds, caffeine, and its flavor as molecules. Throughout the process, pressure is exerted by water from the machine and it pushes materials through the coffee filter to the coffee pot. The smaller molecules like water, flavour and caffeine pass through the filter, but at the same time, the coffee ground can't pass through it as they are too big to pass through it. Holes in the filter can make it possible and coffee ground end up in your coffee. 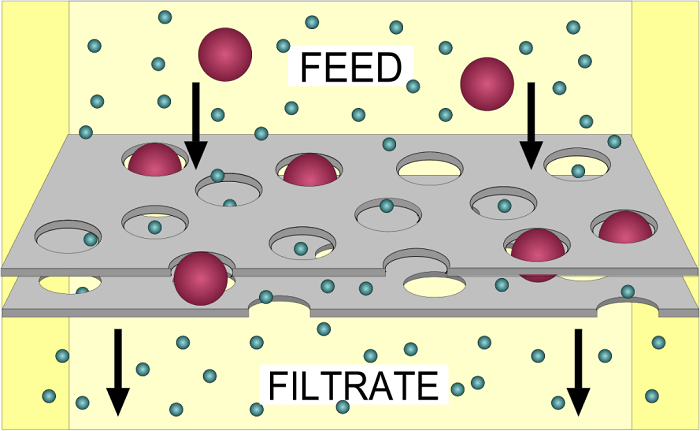
Below are some filtration types which can be used in the separation of substances Cold Filtration Cold filtration can be used at very low temperatures, say by using an ice bath. Substances, like fatty acid particles, are suspended within the mixture as they settle down and then can be filtered easily. Hot Filtration This filtration method is typically used for crystalline compounds that contain impurities. During the process, in this process filtration is done by melting down the crystalline compound, which helps remove the impurities at high temperature. And eventually recrystallizing the currently pure substance. Multilayer Filtration In this process of filtration, a mixture of insoluble solid particles and liquid is poured over the layers, and at the same time, the solid particles are caught during the method, leading to a filtered liquid. Also, this method of multiple layers is used for various materials, such as charcoal, sand, etc., where each material contains different types and sizes of layers of that material. Vacuum Filtration This filtration method is usually used when the substance is filtered in a small volume such as a Hirsch funnel. And with this method of filtration, the Buchner funnel is also used to filter as a piece of equipment used in the laboratory. The main difference between both methods of filtration is that in Hirsch filtration, the plate of the Hirsch funnel is a lot smaller while the walls are outward instead of vertical. Centrifugal Filtration In the Centrifugal filtration method, the substances are to be filtered through high speed. During the horizontal rotation process, the less dense matter is separated from the more dense matter. Gravity Filtration The process of gravity filtration uses gravity to pull liquid through a filter to remove impurities. This can be done through a simple method of filtration by using filter paper in a glass funnel, during the process, insoluble solid particles are taken by filter paper and the rest liquid goes through the gravity pull. Filtration Functions The function of filtration can be used in many ways, for example, to clean river water from impurities. This method is done through sterilization without the use of heat and at the same time, this method of filtration is not enough to kill microorganisms from the water as this can be done through heating. 4. Osmosis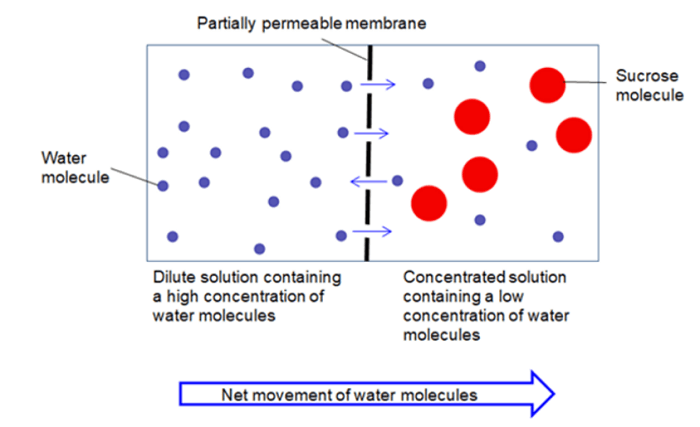
In the process of osmosis, a semi-permeable membrane is there; therefore just the solvent molecules are free to move to match the concentration. More on this, another definition would be osmosis is the movement of solvent particles around a semipermeable membrane from a dilute solution to a concentrated solution. The movement of the solvent is in the direction that tends to match the concentration on both sides of the membrane throughout the process. If you want to see an easy demonstration of osmosis in real life scenario then, soak gummy candies in water, the gel of the candies acts as a semipermeable membrane. Furthermore, red blood cells swelling up when exposed to fresh water and root hairs of plants taking up water are also other such examples.
Next TopicActive Transport
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share









