Nitrogen CycleThe chemical symbol for nitrogen is N, and it is a flavourless and colourless element. Nitrogen is present in the ground beneath us, the water we drink, and the air we breathe. Nitrogen makes up about 78% of the atmosphere, making it the most prevalent element on Earth. All living things, including us, need nitrogen. Insufficient nitrogen hinders plants from growing, which lowers food production. However, too much nitrogen can be harmful to plants. Although nitrogen is essential for our food supply, too much of it can be bad for the environment. 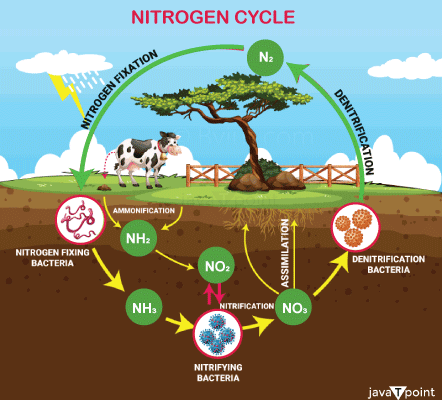
Nitrogen: Why is it Important?It is vital to understand the delicate balance of components required for maintaining life, and nitrogen's position in the environment is no exception. Plants that don't have enough nitrogen grow slowly, turn yellow, and produce smaller fruits and flowers. To promote crop growth, farmers may apply nitrogen-containing fertilisers to their crops. Scientists predict that without nitrogen fertilisers, we could lose up to one-third of the agricultural products that are important for our food supply and other uses. However, we must understand how much nitrogen is required for plant growth because an excess can contaminate streams and harm aquatic life. How Life is Dependent on Nitrogen?A crucial component of the nucleic acids is nitrogen. DNA and RNA are a part of it. Deoxyribonucleic acid, a self-replicating substance that makes up the majority of chromosomes and serves as a genetic information carrier in almost all living things. All living cells contain ribonucleic acid, a nucleic acid that serves as a messenger for DNA, the most significant of all biological molecules and essential to all life. The genetic information, or instructions for assembling a biological form, is carried by DNA. Insufficient nitrogen prevents plants from producing amino acids, which are essential building blocks of many living cells, muscles, and tissues. Plants cannot produce the unique proteins required for plant cells to thrive without amino acids. When there is not enough nitrogen available, plant growth is negatively impacted. Plants that get an excessive amount of nitrogen develop an excess of biomass, or organic matter, such as leaves and stalks, but not enough root system. In extreme circumstances, plants that have absorbed extremely high levels of nitrogen from soils may poison farm animals who consume them. What is Eutrophication and is it Something that can be Avoided?Additionally, extra nitrogen may drain or leak from the soil into subsurface water sources or enter aquatic systems as surface runoff. The buildup of this surplus nitrogen might result in a condition known as eutrophication. When the water is enriched with too much nitrogen, it results in eutrophication, which accelerates the growth of algae and plants. A lake may even turn brilliant green or another colour due to excess nitrogen, along with a "bloom" of the pungent phytoplankton algae. When phytoplankton dies, waterborne microorganisms break them down. Decomposition lowers the amount of dissolved oxygen in the water, which might result in a "dead zone" where there is insufficient oxygen to support the majority of life forms. The lack of oxygen causes organisms in the dead zone to perish. Freshwater lakes and coastal areas where rivers carrying nutrients from agricultural runoff (fertiliser overflow) flow into oceans can also experience these dead zones. Is it possible to stop Eutrophication?Yes. Those in charge of managing water resources can employ a variety of strategies to decrease the harmful effects of algal blooms and eutrophication of water surfaces. To stop algal blooms, reroute surplus nutrients away from lakes and vulnerable coastal zones, and reduce the amounts or combinations of nutrients used in agricultural fertilisers, they can, among other things, use herbicides (chemicals used to kill unwanted plant growth) or algaecides (chemicals used to kill algae). Finding the source of the extra nitrogen and other nutrients, however, can frequently be challenging. It is significantly more difficult to reverse eutrophication once a lake has occurred. Algaecides can be pricey and don't address the excess nitrogen or other nutrients that caused the algae growth in the first place. Bioremediation is a method of purposely altering the food chain in an aquatic ecosystem to lower or regulate the number of phytoplankton, and it is another potential remedy. For instance, water managers can introduce creatures that consume phytoplankton, and by doing so, these species can aid in lowering phytoplankton levels. What is the Nitrogen Cycle?Both living and non-living things, such as the atmosphere, soil, water, plants, animals, and microorganisms, participate in the nitrogen cycle. In order to move through the numerous stages of the cycle, nitrogen must change its forms. Nitrogen can also be found in various forms when used as a fertiliser, such as ammonia, or NH3, which can then be processed further to produce another fertiliser, ammonium nitrate, or NH4NO3. Nitrogen exists as a gas (N2) in the atmosphere, but as nitrogen oxide (NO) and nitrogen dioxide (NO2) in soils. We will now go over each of the five steps of the nitrogen cycle, which are fixation or volatilization, mineralization, nitrification, immobilisation, and denitrification. Because soil microorganisms transform nitrogen gas (N2) into volatile ammonia (NH3), the fixing procedure in this picture is referred to as volatilization. Leaching is the process by which some nitrogen compounds, such as nitrate or NO3, dissolve in water after escaping the soil and potentially contaminating water sources. Stage 1: Fixation of NitrogenDuring this phase, nitrogen is transferred from the atmosphere to the soil. In the Earth's atmosphere, nitrogen gas (N2) is present in enormous quantities. However, this nitrogen is "unavailable" to plants because they must first undergo a transformation in order to use it directly. The N2 must be changed through a procedure known as nitrogen fixation for it to be utilised by plants. Fixation transforms atmospheric nitrogen into forms that plants' root systems can absorb. A small quantity of nitrogen can be fixed when lightning provides the energy required for N2 to interact with oxygen to generate nitrogen oxide, NO, and nitrogen dioxide, NO2. Rain or snow then transports these types of nitrogen into the soil. The commercial technology used to make fertiliser can also fix nitrogen. By mixing atmospheric nitrogen with hydrogen, this nitrogen fixation process creates ammonia (NH3), a type of nitrogen that can be added to soils and utilised by plants. Extreme heat and pressure are used in this technique. The majority of nitrogen is fixed spontaneously by soil microbes. Some bacteria attach to the roots of plants and create beneficial symbiotic partnerships that are beneficial to both the plant and the bacteria. In exchange for the bacteria fixing nitrogen into a form the plant requires, the bacteria receive energy from photosynthesis. In order for the plant to grow, the fixed nitrogen is subsequently transported to other areas of the plant where it is needed to create plant tissues. Without this symbiotic interaction, other bacteria can fix nitrogen in soils or water. These bacteria can also produce nitrogen in forms that living things can use. Stage 2: MineralizationIn the soil, this phase takes place. When nitrogen is transferred from organic substances like manure or plant parts, an inorganic form of nitrogen that plants may use is created. The plant eventually runs out of nutrients, dies, and decomposes. In the second phase of the nitrogen cycle, this becomes crucial. Microorganisms begin to change organic matter into a form of nitrogen that plants may use when they come into contact with organic waste, such as animal manure or decaying plant or animal debris. This process is known as mineralization. All cultivated plants receive the nitrogen they need from the soil, except for legumes (plants having seed pods that split in two, such lentils, beans, peas, or peanuts). Legumes obtain nitrogen through fixation, which takes place in their root nodules as previously mentioned. Ammonia, or NH3, is the initial type of nitrogen created by the mineralization process. The soil's NH3 then combines with water to create NH4, or ammonium. When plants don't obtain their nitrogen from the symbiotic nitrogen-fixing relationship mentioned above, they might utilise the ammonium that is stored in the soils and is accessible for use. Stage 3: NitrificationNitrification, the third stage, also takes place in soils. The process of nitrification converts the ammonia that was generated in the soils during mineralization into nitrites (NO2) and nitrates (NO3). Animals and plants that eat plants can both utilise nitrates. Ammonia can be changed into nitrites by some soil bacteria. Although nitrite cannot be directly utilised by plants or animals, other bacteria can convert nitrites into nitrates, which can. This reaction provides energy to the microorganisms involved in this process. The nitrosomonas and nitrobacter bacteria are the ones we're discussing. Nitrosomonas converts ammonia to nitrites, while Nitrobacter converts nitrites to nitrates. Nitrification is a crucial process for plants because it creates a surplus of nitrogen that is readily available and may be taken by plants through their roots. Stage 4: ImmobilisationThe fourth stage of the nitrogen cycle is immobilisation, also referred to as mineralization in reverse. These two systems work in tandem to control the amount of nitrogen in soils. Similar to plants, soil-dwelling bacteria need nitrogen as a fuel source. When the nitrogen content of plant leftovers from decomposition is insufficient, these soil bacteria take nitrogen from the soil. These types of nitrogen are no longer available to the plants when microbes consume ammonium (NH4+)and nitrate (NO3), which may result in nitrogen shortage, or a lack of nitrogen. Therefore, immobilisation locks up nitrogen in bacteria. However, immobilisation is crucial because it ties up or immobilises nitrogen in microorganisms, which helps control and balance the quantity of nitrogen in soils. Stage 5: DenitrificationIn the denitrification process, which is the fifth stage of the nitrogen cycle, microorganisms convert nitrates into atmospheric nitrogen (N2), releasing nitrogen back into the atmosphere. As a result, soils lose overall nitrogen content because nitrogen gas is released into the atmosphere, which is where our story started. Need for Nitrogen in LifeThe nitrogen cycle within the ecosystem is necessary to maintain healthy, productive ecosystems with an optimum balance of nitrogen. The capacity of plants to create biomass (living matter) depends on the availability of nitrogen. Knowing how the plant-soil nitrogen cycle works can help us make better decisions about what crops to cultivate and where to grow them so that we have an adequate supply of food. Additionally, by better comprehending the nitrogen cycle, we can reduce pollution brought on by excessive soil fertilisation. Some plants might be better at absorbing nitrogen or other nutrients, such phosphorus, from fertiliser. They might even serve as a "buffer" or filter to keep too much fertiliser from entering waterways. A study by Haycock and Pinay found that a riverside area covered with a certain grass (Lolium perenne L.) was able to hold up to 84% of the nitrate and keep it out of the river. When employed as a buffer, poplar trees (Populus italica) retained 99% of the nitrate that entered the subsurface water flow in the winter. As you can see, when soils are deficient in nitrogen, plants suffer, which can be detrimental because excess nitrogen can damage plants and even livestock. Marine life is being suffocated by the breakdown of dead algal blooms, which is causing a major problem with the pollution of our water supplies by excess nitrogen and other nutrients. Farmers and communities must collaborate to increase crops' ability to absorb more nutrients and safely dispose of animal manure waste. The natural plant buffer zones that can absorb nitrogen runoff before it reaches water bodies must also be preserved. But because fewer plants are left to absorb excess nutrients, our current practises of cutting down trees to make way for roads and other building exacerbate this issue. To find the best plant species to flourish in coastal locations and absorb excess nitrogen, we need to conduct further research. We also need to come up with additional solutions or preventative measures for the issue of too much nitrogen entering aquatic environments. We may better grasp how to better conserve Earth's priceless natural resources by striving towards a more thorough understanding of the nitrogen cycle and other cycles at work in the planet's interrelated natural systems.
Next TopicWhat are the Sense Organs?
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share









