Barr Body and Lyon's HypothesisFemales are born with two X chromosomes. Female somatic cells are not engaged in sexual reproduction. According to Lyon's hypothesis, one of the two X chromosomes is inactivated. The dormant X chromosome is referred to as a Barr body. 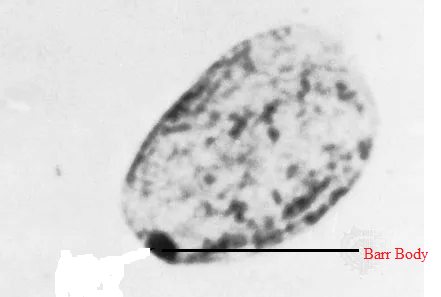
The credit for identifying the technique of X inactivation is given to Mary F. Lyon. She was a British scientist. According to her, one X chromosome of females is deactivated so that no unnecessary information is passed into future generations. This is because males have only one X chromosome, and the inactivation of one X chromosome in females makes it even. This way, the gene expression of both male and female X chromosomes is equal. The reason for X chromosome inactivation is the absence of euchromatin and the presence of heterochromatin in the chromosome. Therefore, inactivated X chromosomes are compressed and cannot be transcribed. The inactivated X chromosomes are compressed and look dark and dense under a microscope. This dense structure is therefore known as the Barr Body. Sex ChromosomesSex chromosomes are different from normal chromosomes because their presence defines particular sex in an organism. They determine whether the person will be male or female. Human and other animals' sex chromosomes are named X and Y chromosomes. In humans, there are a total of 23 pairs of chromosomes. Out of 23, 22 pairs of chromosomes are autosomes, and 2 chromosomes are sex chromosomes. They are represented as X and Y. XX is present in females, and XY is present in males. X and Y Chromosomes - Road To Being Male or FemaleThis road to maleness or femaleness begins during meiosis when a cell splits to generate gametes or sex cells with half the usual number of chromosomes. The X chromosome looks like a giant autosomal chromosome with two arms, one long and one short. The Y chromosome has one long and one extremely small arm. In the meiotic division, the separate gametes containing X and Y chromosomes are produced in males. In contrast, in females, only one type of gametes are formed, containing the X chromosome. Female gametes are called eggs, and eggs have only X chromosomes. Male gametes are called sperm, and sperm can carry either X or Y chromosomes. Eggs fertilized by X-bearing sperm develop into girls (XX), whereas eggs fertilized by Y-bearing sperm develop into males (XY). Unlike paired autosomes, which generally have identical alleles (forms) of the same genes, paired sex chromosomes do not have an identical complement of genetic material. Since the X chromosome is bigger, it contains many more genes than the Y. Sex-linked traits are governed by genes located only on the X chromosome. Men are significantly more likely than women to have recessive sex-linked characteristics such as haemophilia and red-green colour blindness. This is because the male who receives the recessive gene on his X chromosome lacks the Y chromosome allele that would neutralize its effects. For the women to show any phenotype completely, they must inherit the recessive gene on both the X chromosomes. If the female inherits a recessive gene responsible for sex-linked disease on only one of the X chromosomes, she will show the restricted manifestation of the phenotype. This is because of the inactivation of one of the X chromosomes in females, which limits the expression of the gene. The inactivation occurs in each somatic cell of a woman. This inactive X chromosome is visible in the cell nucleus as a tiny, dark-staining structure known as the Barr body. Of course, the consequences of genes carried solely on the Y chromosome are only manifest in men. Most of these genes are known as "maleness determiners," and they are required for the development of the testes in the baby. Several illnesses have been linked to an abnormal number of sex chromosomes. Among the most frequent are Turner's syndrome and Klinefelter's syndrome. MechanismA person with two X chromosomes (like most human females) has one Barr body per somatic cell, whereas a person with one X chromosome (like most human men) has none. The X inactivation centre, or Xic, is located near the centromere and initiates mammalian X-chromosome inactivation. There are a total of twelve genes in the centre. Out of these, seven codes for proteins and five genes code for untranslated RNAs. Two genes, Xist and Tsix, are only known to play a role in the X chromosome inactivation process. From this knowledge, we can say that the centre plays an important role in the process of chromosome inactivation and counting. It makes sure that inactivation occurs randomly and only in those situations when there are already two X chromosomes present. The addition of an additional artificial Xic during early embryogenesis can cause the solitary X present in male cells to be inactivated. Role of Xist and Tsix GenesXist and Tsix's roles look to be adversarial. The absence of Tsix expression on the future inactive X chromosome causes a rise in Xist levels near the Xic. Meanwhile, active X Tsix levels will be maintained in the future, keeping Xist levels low. This change permits Xist to start covering the future dormant chromosome and expand from there. This option appears to be fixed in non-random inactivation, and recent research shows that the maternally transmitted gene may be imprinted. Variations in Xi frequency have been linked to age, pregnancy, and the use of oral contraceptives, menstrual cycle variations, and cancer. This is assumed to be the mechanism of choice, allowing downstream processes to form the Barr body's compact state. The alterations responsible are certain histone modifications and direct DNA modifications. Histone modifications include histone H2A ubiquitination and histone H3 methylation. Direct DNA modifications include CpG methylation. Reactivation of Barr's body is also seen in certain cases, mainly in breast cancer patients. According to some scientific studies, it has been found that the prevalence of Barr bodies is much lower in breast cancer patients as compared to healthy people. The results indicate that there is a reactivation of previously inactivated X chromosomes. Lyons' TheorySixty years ago, British geneticist Mary Frances Lyon (1925-2014) suggested the notion that a unique inactivation process happens in women's sex chromosomes, as well as those of female animals in general, changing them into cellular mosaics. This idea is known as lyonization since then and has been confirmed correct in modern times, leading to intriguing breakthroughs and discoveries. 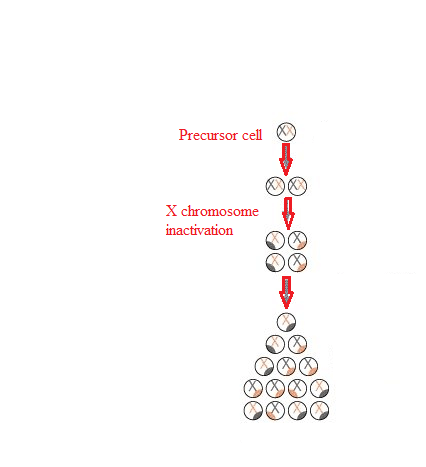
LyonizationDuring the 1950s, there were many studies done on male and female chromosomes. According to one of the studies, it was found that males have a very typical chromosome structure. They have one normal-sized X chromosome inherited from the mother, and the other is a very tiny chromosome called the Y chromosome, inherited from the father. In the case of females, no such tiny chromosome was found, and the two chromosomes were of equal size to the males X chromosomes. Therefore, it was found that females contain two X chromosomes inherited, one from the father and one from the mother. It was then inferred that women had twice as many X chromosome genes as males, which is significant considering that the X chromosome includes over a thousand genes whereas the Y has just roughly 75. As a result, many questions were raised. The major one was whether females express the genes from both the X chromosomes or if there is some kind of compensatory mechanism in women that controls the double expression of genes present on two X chromosomes. In order to answer these questions, Lyon postulated the sex chromosome theory. Lyon's Sex Chromosome TheoryAccording to this theory, one of the two X chromosomes in a female somatic cell is somehow inactivated at the time of interphase, when the genes are transmitted. The inactivated chromosome undergoes a process called heterochromatinization, where the chromosome is condensed, and transcription is inhibited. On the other hand, the second X chromosome and other autosomes do not undergo heterochromatinization; therefore, they are condensed and undergo transcription. It is these inactivated X chromosomes that become Barr body. Lyon's Claims
In conclusion, according to Lyon's hypothesis, mammalian females are mosaics because they contain genes from either one or both of the X chromosomes carried in the cells. This process does not occur in males because they contain only genes from one X chromosome present in their cells and one Y chromosome. Genetic Concepts Related to Lyonization That Are RevolutionaryWhen Mary Lyon first stated her hypothesis on the X chromosome, many people rejected her work. Still, with time, her concept regarding the inner workings of the X chromosome was accepted. Especially since most of the research suggested by her concept (some of it being undertaken) has yielded solid genetic and cytologic data supporting the notion. Once validated, it served as the beginning point for a number of discoveries, some of which are of theoretical biology relevance and others having clinical genetic significance. Thirty years after Lyon's first study on the mechanism, it was discovered that the inactivated X chromosome regulates chromosomal X-inactivation in females. And contrary to popular belief, this is not because a protein is derived from a different chromosome or the same X chromosome. Another remarkable genetic mechanism has been linked to female active X chromosomes. Specifically, another RNA (different from the previously described one) is generated from the Xist gene in this chromosome. However, this occurs from the opposite strand of the double helix of the gene ? and in the opposite orientation ? to that which is transcribed in the inactive chromosome. This RNA is known as Tsix, which is Xist reversed. It is much longer than the Xist; it has 40,000 bases, and its synthesis in the active X chromosome inhibits the Xist RNA from being synthesized with the inactivating function. It is also one of the first cases in which two different RNAs were observed to be transcribed from a single gene: one in one direction of the gene's double helix and the other in the opposite direction, and where one of the RNAs interferes with the other: if the Tsix impedes the Xist synthesis, it would be an anti-Xist. Lyonization's Clinical ImplicationsLyonization, which happens throughout the development of women and mammalian females in general, may potentially have therapeutic implications. These include;
However, in rare cases, having an X chromosome with a functional gene opposite a mutant gene in the other X chromosome of a woman does not protect her against the illness. This happens, for example, with Rett syndrome, which primarily affects women and is characterized by symptoms such as a severe cognitive deficit?a severe type of autism?that worsens over time. A mutation causes this condition in a gene on the X chromosome that produces a protein. The protein binds to DNA in several places and affects the expression of numerous other genes. This happens mostly in the brain. The organism does not survive when women have a mutation in both of their X chromosomes and males have a mutation in their only X chromosome present in the cell. On the other hand, the heterozygous organisms having one X chromosome mutated and the other X chromosome normal are perfectly viable. In this instance, just half of the patients' brain cells express the normal gene ? the cells that have inactivated the mutant X. The other half, however, does not make the protein since the X was inactivated with the normal gene. Half of the protein produced by nerve cells is insufficient for appropriate brain function.
Next TopicComponents of a Balanced Diet
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share









