Atomic Radius DefinitionAtomic radius is defined as the measure of the size of its atom, which is one of the basic properties of an element. The atomic radius is an important factor in defining an element's chemical and physical properties. Atomic radius is difficult to measure as an atom has no physical boundaries. So, we cannot measure the distance from the nucleus exactly. In this article, we will explore atomic radius, how it is estimated, its different types, and how it varies across the periodic table. 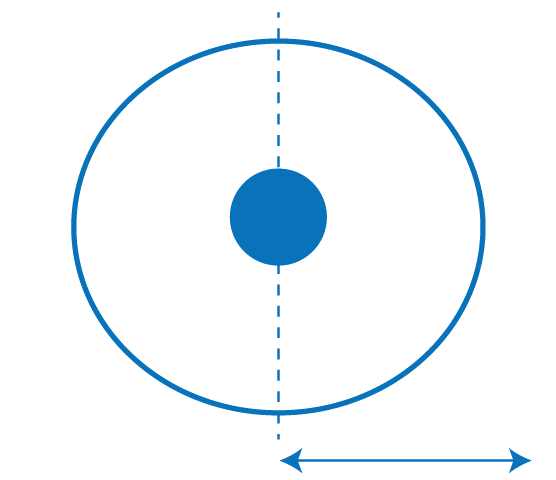
Numerous techniques, including X-ray Crystallography and Spectroscopy, can be used to calculate the atomic radius. When a crystal is subjected to an X-ray bombardment in X-ray crystallography, the diffraction pattern calculates the distances between its atoms. The electromagnetic radiation particles receive or emit is measured in spectroscopy to ascertain their energy levels and atomic structure. One picometer (pm), equal to 10-12 meters, is commonly used to indicate the atomic radius. The atomic radius varies across the periodic table due to the differences in the atomic properties, like the number of protons, neutrons, and electrons in the atoms. Generally, as you move from left to right across a period, the atomic radius decreases due to the increasing nuclear charge, which pulls the electrons closer to the nucleus. Conversely, the atomic radius increases as you travel downside a group because more electrons are added to the shell. The size of an atom also affects its chemical and physical properties. For example, smaller atoms generally have higher ionization energies, requiring more energy to remove an electron from the bit. This is because, in this case, the electrons are situated nearer to the nucleus. The force of attraction between the positively charged nucleus and negatively charged electrons is stronger, holding them tightly. On the other hand, larger atoms have lower ionization energies because the electrons are located away from the nucleus, and the attraction between them is weaker. 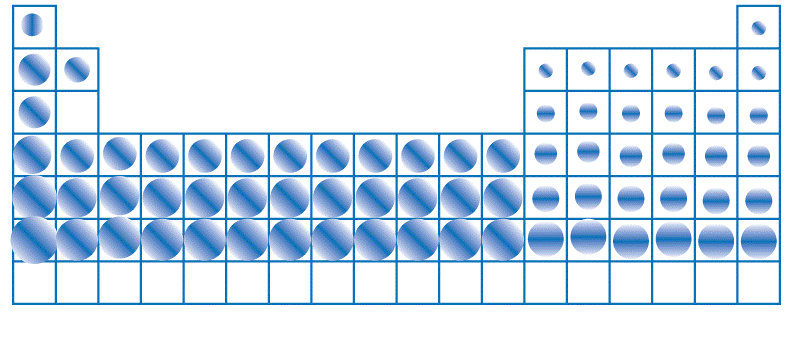
The atomic radius also affects the reaction characteristics of an element. For example, the alkali metals in Group 1 of the periodic table have the largest atomic radii in their respective periods. This means they are highly reactive because they have low ionization energy, allowing them to lose an electron and form positive ions easily. Conversely, the noble gases in Group 18 have the smallest atomic radii in their respective periods, making them highly unreactive because they have high ionization energy, making removing an electron from them difficult. There are two main types of atomic radius: the Covalent Radius and the Metallic Radius. Every kind of atomic radius describes a different aspect of an atom's size and is determined by various methods. The covalent and metallic radii are two main types of atomic radii that describe different aspects of an atom's size. The covalent radius is used for non-metals and is calculated by scaling the bond length in a group of atoms. In contrast, the metallic radius is used for metals and calculated by scaling the distance between two atoms in a metallic crystal lattice. Studying the different types of atomic radii is necessary to understand and explain the chemical and physical properties of elements and their interactions with other substances. Let us discuss them in brief:
In addition to the covalent and metallic radii, other types of atomic radii are used to describe specific properties of an atom. For example, the Van Der Waals radius describes the size of an atom when it is not bonded to another atom, and the ionic radius describes the size of an ion when it has lost or gained electrons. Some Solved Questions on Atomic Radius1. Which element has the largest atomic radius: carbon, nitrogen, or oxygen? Explain with a valid reason. Solution: The atomic radius generally increases as you move down a group and decreases as you move across a period from left to right. Carbon, nitrogen, and oxygen are all in the same period, but oxygen is farther to the right than nitrogen, which is farther to the right than carbon. Therefore, oxygen has the largest atomic radius of the three elements. 2. What is the covalent radius of a hydrogen atom in an H2 molecule, given that the bond length is 74 pm? Explain with a valid reason. Solution: The covalent radius is half the bond length, so the covalent radius of a hydrogen atom in an H2 molecule would be 74 pm / 2 = 37 pm. 3. How does the metallic radius of calcium compare to the metallic radius of barium? Explain with a valid reason. Solution: Calcium and barium are in the same periodic table (Group 2). As you scroll down a group, the metallic radius generally becomes larger. Therefore, barium has a larger metallic radius than calcium. 4. Which ion has the greater ionic radius: Ca2+ or K+? Explain with a valid reason. Solution: Ca2+ and K+ have the same electron configuration as their nearest noble gas, but Ca2+ has two fewer electrons than K+. Since the ionic radius generally decreases as the number of electrons decreases, Ca2+ has a smaller ionic radius than K+. 5. Which element has the smallest atomic radius: fluorine, chlorine, or bromine? Explain with a valid reason. Solution: Fluorine, chlorine, and bromine are all in the same period, but fluorine is farther to the left than chlorine, which is farther to the left than bromine. Therefore, fluorine has the smallest atomic radius of the three elements. 6. What is the covalent radius of a chlorine atom in a Cl2 molecule, given that the bond length is 198 pm? Explain with a valid reason. Solution: The covalent radius is half the bond length, so the covalent radius of a chlorine atom in a Cl2 molecule would be 198 pm / 2 = 99 pm. 7. Which ion has the greater ionic radius: Li+ or Be2+? Explain with a valid reason. Solution: Li+ and Be2+ have the same electron configuration as their nearest noble gas, but Be2+ has two fewer electrons than Li+. Since the ionic radius generally decreases as the number of electrons decreases, Be2+ has a smaller ionic radius than Li+. 8. What is the metallic radius of a copper atom in a copper crystal lattice, given that the distance between adjacent copper atoms is 256 pm? Explain with a valid reason. Solution: The metallic radius is half the distance between adjacent atoms, so the metallic radius of a copper atom in a copper crystal lattice would be 256 pm / 2 = 128 pm. 9. How does the covalent radius of nitrogen compare to the covalent radius of oxygen? Explain with a valid reason. Solution: Nitrogen and oxygen are in the periodic table's same period (Period 2). The covalent radius generally decreases as you move across a period from left to right. Therefore, oxygen has a smaller covalent radius than nitrogen. 10. Which element has the largest atomic radius: lithium, sodium, or potassium? Explain with a valid reason. Solution: Lithium, sodium, and potassium are all in the same group (Group 1) of the periodic table. As you move down a group, the atomic radius generally increases. Therefore, potassium has the largest atomic radius of the three elements. ConclusionThe atomic radius is an important property of an element that influences its chemical and physical properties. It is the length between the nucleus and the last shell of the atom in which electrons are present at an instant of time and can be measured using various techniques such as X-ray crystallography and spectroscopy. The atomic radius varies across the periodic table due to differences in the number of protons, neutrons, and electrons in the atoms. Understanding the atomic radius can help us understand the physical and chemical properties and characteristics of atoms of each element and their reactivity to other substances.
Next TopicBed Making Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










