Monosaccharide DefinitionA monosaccharide is a basic form as well as the simplest type of carbohydrate. Monosaccharides can be connected through glycosidic bonds to form immense carbohydrates, like oligosaccharides or polysaccharides. A disaccharide is an oligosaccharide formed of simply two monosaccharides. When over 20 monosaccharides are linked by glycosidic bonds, an oligosaccharide transforms into a polysaccharide. This article explains the structure and functions of monosaccharides, along with providing examples. An Outline of MonosaccharideWhen it comes to the world of sugars, monosaccharides are the simplest sugar which is derived from two Greek words: "mono," meaning "one," and "saccharide," meaning "sugar molecule." Monosaccharides cannot be broken down further and are defined as a type of sugar consisting of only one sugar molecule. Carbohydrates, on the other hand, consist of saccharide molecules which include not just monosaccharides but also disaccharides and polysaccharides. It's important to note that although all carbohydrates come from monosaccharides, not all monosaccharides can be classified as carbohydrates. It's worth mentioning that carbohydrates are an essential macronutrient for our bodies since they provide energy to keep us going throughout the day. Each individual carbohydrate consists of its own unique set of monosaccharides with their specific functions in our body. Structure of MonosaccharideMonosaccharides have the formula (CH2O)n. The central carbon molecule bonds to two hydrogens and one oxygen, and the oxygen also bonds to hydrogen to form a hydroxyl group. Carbon molecules can bond because of carbon's ability to form four bonds. When one of the carbons in the chain forms a double bond with oxygen, it creates a carbonyl group. Monosaccharides can be classified as either aldose or ketose based on the location of their carbonyl group. If the carbonyl is at the end of the chain, it's an aldose; if it's in the middle, it's a ketose. Glucose ChainGlucose is a simple monosaccharide composed of six carbons. It belongs to the aldose family due to its carbonyl group being at the end of the molecule. Monosaccharides with over five carbons form ring in water solutions. The fifth carbon's hydroxyl group reacts with the first carbon. The hydroxyl group loses a hydrogen atom when bonding with the first carbon. When the second bond between a carbon and oxygen molecule is broken, the double-bonded oxygen on the first carbon forms a stable ring of carbons by bonding with a new hydrogen. 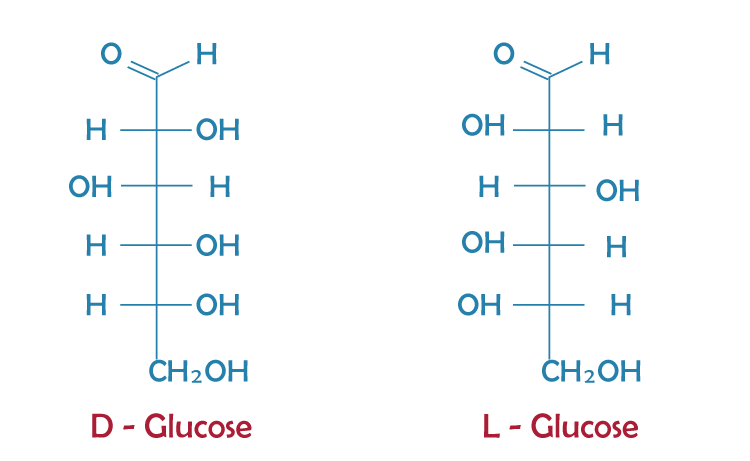
Monosaccharide TypesThere are 20 types of monosaccharides in nature, which can be divided into two categories. 1. Aldoses:Aldoses are a type of monosaccharide that has an aldehyde functional group in their molecular structure. They are classified based on the number of carbon atoms they contain, and the aldehyde group is always located at the end of the monosaccharide chain due to only having one vacant orbital. 2. Ketoses:Ketoses are monosaccharides that have a ketone functional group in their molecular structure. They can be classified based on the number of carbon atoms they contain, such as triose, tetrose and pentose. The placement of the ketone group can vary since it has only divalent valence. Some Examples of Monosaccharides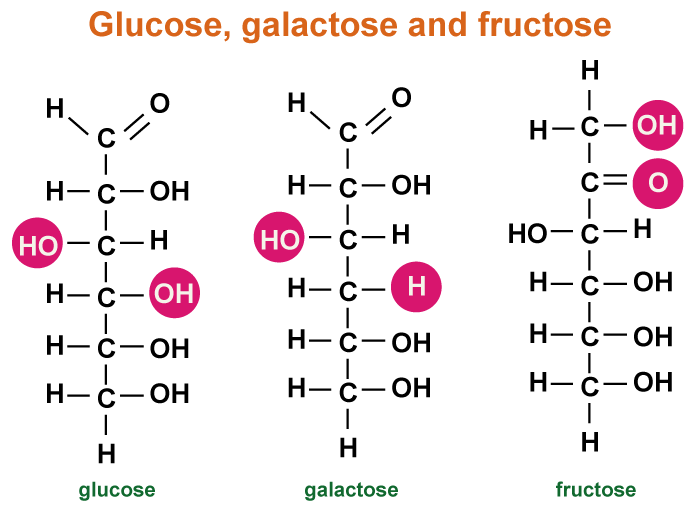
a) GlucoseGlucose is a vital sugar that provides energy and structure to organisms. It can be broken down through glycolysis to provide energy. When the cell doesn't need more energy, excess glucose can be stored by combining it with other monosaccharides. Glucose can be stored as starch in plants by combining it with other monosaccharides. Later, the starch can be disassembled and used for energy. Monosaccharides can also form fiber-like polysaccharides when connected in long chains. Plants produce cellulose, a common molecule if weighed in total, it can weigh millions of tons, to create cell walls that provide structure and rigidity. The combination of monosaccharides forms long chains that give plants their structure. b) FructoseFructose is a ketose found in sweet fruits like honey, mangoes, pears, sugar cane, apples, and peaches. It's also known as fruit sugar and commonly occurs alongside glucose. Fructose is found in both sucrose and inulin. When combined with glucose, it forms glycosidic bonds in sucrose. It also forms oligosaccharides when combined with other monosaccharides. Fructose and glucose have the same formula but have different structures. Fructose is a ketose, not an aldose like glucose because the carbonyl group is at the second carbon instead of the end of the molecule. Fructose has six carbons with a hydroxyl group attached, just like glucose. Fructose has a slightly different-shaped ring due to the position of its double-bonded oxygen. Fructose is a monosaccharide that can be combined with other monosaccharides to form oligosaccharides, such as sucrose, which is made up of one fructose molecule and one glucose molecule. Further, Cells use specific enzymes to catalyze most reactions. Monosaccharides with different shapes require specific enzymes for their breakdown. c) GalactoseGalactose is a simple sugar found in many organisms, particularly mammals. It is used by mammals to provide energy to their offspring through milk. When Galactose combines with glucose, it forms the disaccharide lactose. Newborn mammals produce enzymes to break the bonds in lactose, which contains a lot of energy. However, these enzymes are lost once they are weaned off their mother's milk. Humans are unique among mammals in their consumption of milk as adults, which has led to the development of distinctive enzyme functions. The ability to digest lactose varies among populations based on their milk consumption habits. Those who consume a lot of milk can typically digest lactose throughout their lives, while those who do not drink milk after weaning are more likely to be lactose intolerant. Lactose intolerance causes abdominal cramps and diarrhea due to toxins produced by bacteria digesting excess lactose. This raises the solutes in the intestines, causing them to retain more water for a stable pH. 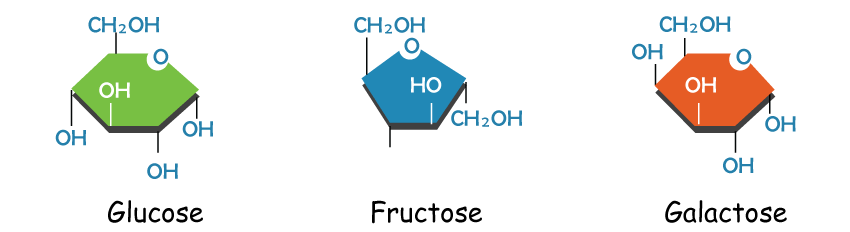
Monosaccharide ConfigurationWhen writing about glucose and fructose, it's essential to use their correct names. For optically active molecules like these, there are enantiomers to consider. It means that before the names of these molecules, you should mention L-(-) and L-(+), respectively. Similarly, for D-(+) and D-(-), you should also include this information before their names. The letters 'D' and 'L' indicate the configuration of the -OH group at the end of monosaccharide carbon chains, while '+' and '-' denote their optical rotation positions. Monosaccharide FunctionMonosaccharides serve various roles in cells, primarily as a source of energy. Glucose is the most commonly used monosaccharide for producing and storing energy in organisms by breaking down its bonds. Plants create cellulose to form cellular structures, while bacteria produce a similar cell wall from different polysaccharides. Animal cells have a complex matrix of polysaccharides made from smaller monosaccharides. ConclusionMonosaccharides are the simplest form of carbohydrate compounds that cannot be further hydrolyzed. They are composed of a single sugar molecule and can be classified into two main categories based on their functional groups. If the monosaccharide contains an aldehyde group, it is known as an aldose, while if it contains a keto group, it is known as a ketose. Monosaccharides are colorless and crystalline solids that readily dissolve in water. Some monosaccharides have a sugary taste and are often used as sweeteners in food production. FAQs1. What are the Main Sources of Galactose Monosaccharides? Galactose is a monosaccharide similar to glucose and fructose in formula but different in geometry. It can be found in various food sources.
2. Why are monosaccharides essential? Monosaccharides serve numerous purposes in cells, primarily as a source of energy. The breakdown of glucose releases energy stored in the bonds and is used by most organisms to generate energy. 3. What occurs when glucose is exposed to an alkali? Glucose can be treated with either a concentrated or dilute solution of an alkali, resulting in different outcomes. Concentrated solutions cause glucose to turn yellow and brown before forming a brown-resinous mass. However, dilute solutions cause reversible isomerization, leading to an equilibrium mixture of D-glucose, D-fructose, and D-mannose.
Next TopicAdjacent Angles Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










