Diffusion Definition BiologyDiffusion is the process of the transfer of molecules from a region with a higher concentration of molecules to an area with a lower concentration. 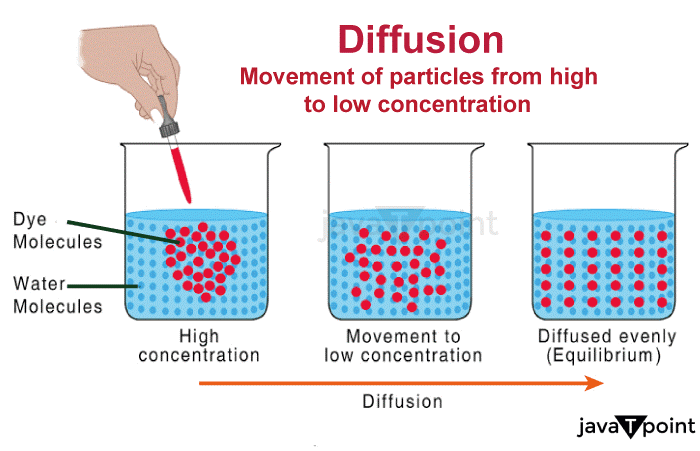
Meaning of DiffusionThe overall movement of any matter such as atoms, ions, particles, or energy from one region of higher concentration to another region of lower concentration is known as diffusion. Diffusion is stimulated by a gradient in the Gibbs free energy or chemical potential.Diffusion is the term used to describe how wave packets spread in quantum physics. In the most basic illustration, as time passes, a Gaussian wave packet's energy diffuses as it moves along the spatial dimensions. It is possible for atoms to diffuse "uphill" via a region of low concentration to a region of higher concentration, similar to spinodal breakdown. Due to the diffusing entity's inherent inconsistency, diffusion is an unpredictable procedure that can be utilized to resemble a variety of unpredictable situations that occur in the actual world. The term "diffusion" is used frequently across a variety of disciplines, including physics (diffusion of particles), biological sciences, chemistry, sociology, statistics, economics, data science, and financial management (diffusion of people, ideas, data, and price values). These fields simulate stochastic systems using the same or related mathematical models derived from statistical physics. They all adhere to the fundamental concept of diffusion, which implies that a substance or collection spreads from a site where there is a higher concentration of that substance or collection. A gradient is a change in a quantity's value caused by a change in another variable, typically distance, such as concentration, pressure, or temperature. The terms "concentration gradient," "pressure gradient," and "temperature gradient" are used to describe changes in concentration, pressure, and temperature over a given distance. Diffusion is distinguished by the fact that mixing or mass movement occurs without the need for the directed bulk motion because it relies on random particle walk. Advection is a property of bulk motion or bulk flow. Convection is the scientific term given to the result of combining the two transport phenomena. Types of DiffusionDiffusion is frequently used in several disciplines, including physics, chemistry, and biology. Diffusion can be divided into two main categories: simple diffusion and facilitated diffusion: 1. Simple diffusionAn action where the substance passes across a semipermeable barrier or solution without the aid of transport proteins. In the cytoplasm, for instance, bacteria use simple diffusion to supply minute nutrients, water, and oxygen. 2. Facilitated diffusionFacilitated diffusion occurs when molecules passively move from a location of higher levels of concentration to a region of less concentration through the cell membrane using a carrier molecule.
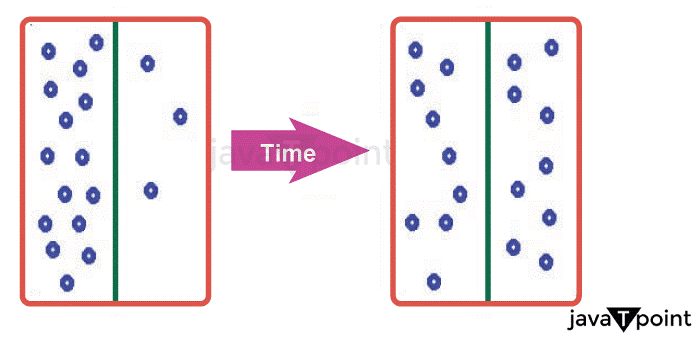
Factors Affecting DiffusionThe medium by which a substance diffuses, the dimensions of the molecules passing through, the temperature of the solution, and the separation between collisions are only a few of the factors that affect diffusion rates. While diffusion occurs in an environment of a chemical containing a gradient in concentration, several factors affect the diffusion rate. A few things influence the diffusion process, and they each have an impact on the speed and scope of dissemination, both individually and collectively. These elements consist of:
Difference between Diffusion and Bulk FlowA "bulk flow" is when a whole body moves or flows as a result of a pressure gradient, for as when water is released from a faucet. When there is no net movement of matter, "diffusion" refers to the slow movement or dispersion of concentration caused by a concentration gradient within a body. Human breathing is an illustration of a mechanism that combines diffusion and bulk motion. The "bulk flow" process comes first. As the process of external respiration begins, the thoracic cavity, which houses the lungs, enlarges. The alveoli's volume increases as a result of this expansion, which lowers their internal pressure. As a result, there is a pressure difference between the alveoli, which have a comparatively low pressure, and the air beyond the body, which is relatively high pressure. Air flows down the pressure gradient through the lungs' airways and into the alveoli until the air pressure and the pressure in the alveoli are equal; once there is no longer a pressure gradient, bulk air flow ceases to occur. The second process is one of "diffusion". The oxygen content of the air entering the alveoli is higher than that of the "stale" air already there. A gradient of oxygen concentration between the blood in the alveoli's surrounding capillaries and the air inside creates by an upsurge in oxygen concentration. Following that, oxygen diffuses into the blood along the gradient of concentration. The alveoli's reduced carbon dioxide concentration is the other effect of air entering the alveoli. There is a concentration gradient that enables carbon dioxide to go from bloodstream into the alveoli because fresh air has a considerably smaller amount of carbon dioxide than does the blood in the body. Another "bulk flow" procedure is the third. The blood is then moved throughout the body by the heart's pumping function. The volume of the heart's left ventricle reduces when it contracts, which raises the ventricular pressure. Due to the pressure differential created between the capillaries and the heart as a result, blood flows via blood arteries in a bulk flow down the gradient. 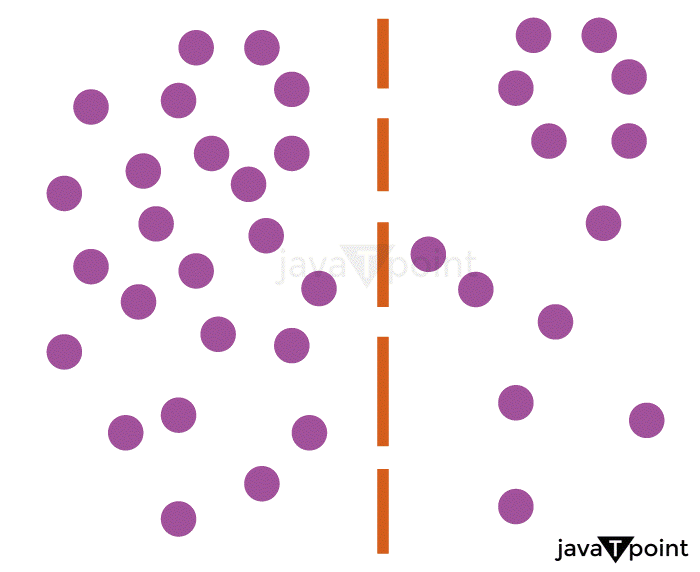
Importance of DiffusionThe various life processes all entail the crucial mechanism of diffusion. It is the overall dispersion of particles, ions, molecules, solutions, etc., as was already mentioned. Diffusion is crucial to the movement of molecules in all living things as they are metabolized by cells. The following are some reasons why diffusion is significant:
Examples of Diffusion
Next TopicDigital Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










