Molarity DefinitionIn chemistry, molarity is a unit of concentration used to represent how much a solute is present in a solution. It is described as the number of solute molecules per liter of solution. The symbol for molarity is "M". It is also called molar concentration and is typically used in the presence of dilute solutions, meaning that the solute concentration is relatively low compared to the solvent. It is simple to convert between molarity & moles, making molarity one of the most advantageous concentration units. This is because the volume of the solution is in liters, a unit of measurement familiar to most people. Furthermore, the amount of solute present in a solution, expressed in moles, is proportionate to molarity. Accordingly, the molarity of a solution will likewise double if the solute concentration is doubled from one mole to two moles. 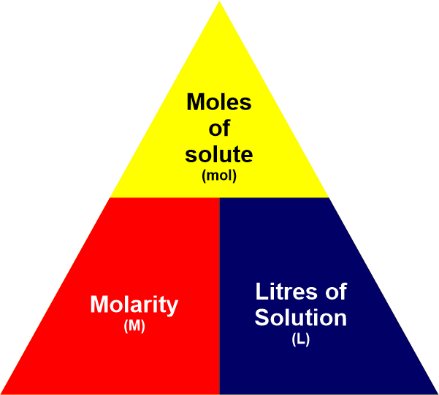
In the laboratory, molarity is frequently employed while creating solutions. Many chemical reactions require a specific concentration of reactants to proceed efficiently. Using molarity, scientists and researchers can easily prepare solutions of a specific concentration using a serial dilution technique. This involves diluting a concentrated solution with a solvent to achieve the desired molarity. Molarity is also used in biochemistry to gauge the concentration of enzymes and other biomolecules in a solution, which is another significant application of the concept. Enzymes are biological catalysts that speed up chemical reactions, and their activity is often measured by determining the rate at which a substrate is converted to a product. The enzyme activity depends on the concentration of the enzyme in the solution, which can be determined by measuring the molarity of the enzyme. What exactly is molarity in chemistry?The molarity is the sum of all moles of solute in a given solution per liter. Additionally, it is also spelled "molar". It is symbolized by the letter "M," and the unit is expressed in mol/L, mol/dm^3, or mol/m^3. For one mole of the solute to dissolve in one liter of the solution, the molarity of the solution is stated to be one molar. While not the amount of solvent, the volume of a solution is determined by its volume. A disadvantage of molarity is that due to thermal expansion, the volume of most solutions varies with little temperature dependence. Molarity is, therefore, only sometimes employed in thermodynamics. 
Molecularity UnitMol/m^3 is the coherence unit for molarity or molar concentration in the International System of Units (SI). However, this is problematic for most laboratory applications, and most chemical literature typically utilizes mol/dm^3. This corresponds to mol/L. This traditional unit is frequently symbolized by the letter M, with the SI prefix mega if necessary to indicate sub-multiples. As an example: mol/m^3 = 10^?3 mol/dm^3 = 10^?3 mol/L = 10^?3 M = 1 mM = 1 mmol/L. The terms millimolar and micromolar stand for mM (10^-3mol/L) and ?M (10^-6mol/L), respectively. Molarity FormulaBy definition, the molarity of a solution is defined as the total number of moles of solute present in a particular volume of solution. Thus, the formula of molarity is described as follows:M=n/V Here, the solution's molality is indicated by the letter M, n refers to the number of moles present in the solute, and V represents the volume of the solution in terms of liters. Thus, it establishes the formula as below: 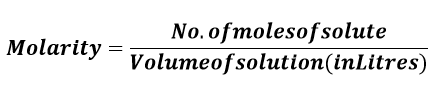
Solved ExampleConsider a mixture made with 15 g of sodium sulfate. The solution is 125 ml in volume. The specified sodium sulfate solution's molarity needs to be determined. Solution:Sodium sulfate's chemical name is Na2SO4, and H2O is the molecular formula for water. So, the molecular mass of sodium sulfate can be calculated as follows: M= 23×2+32+16×4= 142 Now, the number of moles of sodium sulfate needed to solve the problem can be calculated using the formula: N = mass in grams/molecular weight Thus, N =15/ 142= 0.106 In light of this, the solution's volume is 125 ml (as already given). When the figures mentioned above are expressed in liters, it becomes as below: Volume = 125/ 1000 = 0.125 L Now, we determine the molarity of the provided solution using the formula provided below: Molarity= No. of moles of solute/ solution volume (in lit.) By putting the values in the above formula, we obtain the molarity as below: Molarity = 0.016/ 0.125, resulting in 0.85 mol/L. Thus, the solution molarity is 0.85M or 0.85 mol/L. ConclusionIn conclusion, molarity is a widely used unit of concentration in chemistry that is easy to understand and use. It is particularly important in analytical chemistry and biochemistry, where it is used to prepare solutions of a specific concentration and to measure the concentration of enzymes and other biomolecules. Understanding the concept of molarity is crucial for scientists and researchers to interpret results from experiments accurately and to make informed decisions.
Next TopicNon-Verbal Communication Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










