Haemoglobin DefinitionHaemoglobin is a word for a category of protein molecules found in red blood cells that transport oxygen that comes from the lungs to the body's tissues & carbon dioxide back to the lungs. The four protein molecules that make up the structure are referred to as globin chains, and they are all joined together. In a typical adult human body, two alpha-globin and two beta-globin chains are present; however, in newborns, beta-globin chains are absent, and baby haemoglobin is composed of two alpha and two gamma-globin chains. 
Gamma globin develops into beta globin as people get older, forming the structure of adult haemoglobin. Moreover, globin has a key iron-containing porphyrin complex called the heme group, which includes an iron atom that is essential for the movement of oxygen and carbon dioxide in human blood. Blood's red colour is also a result of iron in haemoglobin. Definition of HaemoglobinHaemoglobin is an iron-containing protein found in the red blood cells (erythrocytes) of vertebrates and the blood of many other animals that carries oxygen to the tissues. As oxygen and haemoglobin combine, their relationship is fragile and reversible. It is known as oxyhaemoglobin and is brilliant red when it is oxygenated; it is purplish blue when it is decreased. Red blood cell-producing cells in the bone marrow produce haemoglobin as they grow. Haemoglobin is broken down when red blood cells die. Iron is recovered, transferred to the bone marrow by proteins known as transferrins, and used to make new red blood cells. The remaining haemoglobin serves as the building block for bilirubin, a substance that is expelled into the bile and gives the stools their distinctive yellow-brown color. A globin group is surrounded by four heme groups, which together create a tetrahedral shape in each haemoglobin molecule. Heme is made up of porphyrin, a chemical structure with rings that have an iron atom connected to it. Heme makes up just 4% of the weight of the whole molecule. When the blood circulates between the lungs and the tissues, the iron atom is what holds oxygen in place. Each haemoglobin molecule contains four iron atoms, and as a result, it has the capacity to bind four oxygen molecules. The polypeptide chains of globin are connected in two pairs. The severe genetic type of anemia known as sickle cell anemia, in which the cells take on the appearance of a crescent moon when oxygen is scarce, is characterised by the presence of haemoglobin S, a different form of haemoglobin. The aberrant sickle-shaped cells may lodge themselves in tiny blood arteries before they pass away, preventing the blood flow and perhaps causing tissue injury. While individuals of Middle Eastern, Mediterranean, or Indian heritage may also have sickled illness, those of African descent are more likely to have the characteristic. History of HaemoglobinJohn Friedrich Engelhart found in 1825 that the iron to protein proportion in the haemoglobins of various animals is the same. He determined the molecular mass of haemoglobin to be n 16000 (n = numbers of iron atoms per haemoglobin, currently known to be 4), the first measurement of a protein's molecular mass, using the known atomic mass of iron. The idea that any molecule could be so large garnered a lot of ridicule from other scientists at the time for being a "hasty conclusion." Gilbert Smithson Adair measured the osmotic pressure of liquids containing haemoglobin and used the data to validate Engelhart's findings in 1925. The oxygen-carrying capacity of haemoglobin was first reported by H�nefeld in 1840, despite the fact that blood has at least been known to do so since 1794. Otto Funke, a German scientist, detailed the formation of haemoglobin crystals in a series of publications he wrote in 1851. He said this was accomplished by slowly evaporating the solvent from the protein solution after dilutions of red blood cells with clean water, alcohol, or ether. A few years later, Felix Hoppe-Seyler explained how haemoglobin may reversibly oxygenate. Protein structures may now be sequenced thanks to the invention of X-ray crystallography. The molecular makeup of haemoglobin was discovered by Max Perutz in 1959. Along with John Kendrew, who analyzed the globular protein myoglobin, he was awarded the 1962 Nobel Prize in Chemistry for this achievement. Claude Bernard, a French scientist, explained haemoglobin's function in the blood. The terms heme and globin were combined to create the name haemoglobin, which reflects the fact that each haemoglobin component is a globular protein with an embedded heme group. One iron atom is present in each heme group, and using the dipole forces produced by ions, it may bind one oxygen molecule. There are four of these components in the most prevalent kind of haemoglobin found in animals. Function of HaemoglobinThe primary purpose of haemoglobin is to provide oxygen to diverse tissues. Oxygen binds to Hb in a cooperative manner. The changeover between the reduced oxygen attraction T state (tense) and the high oxygen attraction R state is what causes the interaction and releasing of oxygen from Hb in the tissues as well as the lungs, respectively (Relaxed). 1. Transporting Oxygen
2. Carbon Dioxide Transportation
Hence, the binding of oxygen may be impacted by even a very low CO concentration. Inhaling CO-rich air might thus result in headaches, nausea, or even coma. In heavy smokers, it may inhibit 20% of the active oxygen binding sites. Structure of HaemoglobinThe molecular composition of haemoglobin was first identified by Max Perutz in 1959. Tetrameric proteins make up haemoglobin. '?' and '?'polypeptide chains, each with two subunits, make up the major kind of haemoglobin seen in adults. Each polypeptide chain has a heme prosthetic group connecting it to the next. Four chains of amino acids make up the structure of haemoglobin. Heme is a component of each of these chains. Iron is present in the chemical heme. The movement of oxygen through the bloodstream is one of heme's primary jobs. 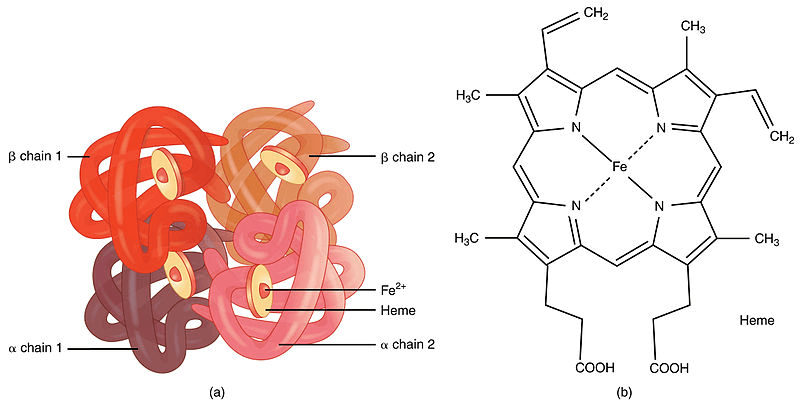
The shape of RBCs is determined by haemoglobin. RBCs often resemble donuts but lack a hole in the core and instead have a thin centre. RBCs' shape and capacity to carry oxygen and carbon dioxide are both impacted by aberrant haemoglobin types, which include:
Structure of HemeHaemoglobin is a multi-subunit globular protein that demonstrates the quaternary structure common to many such proteins. The majority of the amino acids in haemoglobin organize into alpha helices, which are joined by brief non-helical segments. Haemoglobin's quaternary structure is made up of its four subunits, which are arranged approximately in a tetrahedral form. Hydrogen bonds help stabilise the helical parts of this protein, which in turn creates attractions inside the molecule and enables each polypeptide chain to fold into a certain shape. 
Four globular protein components are assembled to form the haemoglobin molecule in the majority of animals. Each component is made up of a protein chain that is closely linked to a prosthetic heme group that isn't made of protein. Each protein chain forms a series of structural alpha-helix segments that are coupled to one another in a globin fold configuration. This configuration has a folding motif with other heme/globin proteins like myoglobin, which is why it has been given that name. This folding pattern has a pocket that firmly bonds the heme group. A porphyrin, a heterocyclic ring, is what holds an iron (Fe) ion inside a heme group. Four pyrrole molecules are cyclically connected to one another (via methine bridges) to form this porphyrin ring, which is bonded with an iron ion in the middle. The four nitrogen atoms are located in the ring's core and are all aligned in one plane coordinate with the iron ion, which is where oxygen is bound. Via the N atoms of the imidazole ring of the F8 histidine residue (also known as the proximal histidine), which is located below the porphyrin ring, the heme is firmly (covalently) attached to the globular protein. The octahedral group of six ligands may be completed by a sixth location that can reversibly bind oxygen by a coordinate covalent connection. Because of this reversible bonding with oxygen, hemoglobin effectively carries oxygen throughout the body. Oxygen bonds to iron in an arrangement known as an "end-on bent" geometry, in which one oxygen atom attaches to iron while the other protrudes at an angle. Unbound oxygen forms a deformed octahedron when it is replaced by a water molecule with extremely weak bonds. Although being transported by haemoglobin, carbon dioxide binds to the amine groups of the protein chains linked to the heme groups instead of competing with oxygen for the iron-binding sites. Ferrihaemoglobin (methaemoglobin) (Fe3+) cannot bind oxygen even though the iron ion may exist in either the ferrous Fe2+ or the ferric Fe3+ state. Since oxygen momentarily oxidizes (Fe2+) to (Fe3+) while also temporarily converting into the superoxide ion during binding, iron must be in the +2-oxidation state. The iron in haemoglobin will continue to be oxidised and unable to bind oxygen if the superoxide ion associated with Fe3+ is protonated. Under these circumstances, methemoglobin may ultimately be reactivated by decreasing the iron core thanks to the enzyme methemoglobin reductase. Haemoglobin A, a tetramer (which has four component proteins) that consists of two ?and two ? non-covalently bound subunits with 141 and 146 amino acid residues apiece, is the most prevalent kind of haemoglobin in adult humans. The symbol for this is ?2?2. The subunits are around the same size and have a similar structural makeup. The tetramer has a total molecular weight of around 64,000 daltons (64,458 g/mol)and each subunit has a molecular weight of about 16,000 daltons, making 1 g/dL equal to 0.1551 mmol/L. The haemoglobin molecule that has been examined the most extensively is haemoglobin A. The haemoglobin molecule in newborn humans is composed of two ?chains, two?chains, and two chains. As the baby develops, the ?shackles are eventually replaced by?chains. The hydrophobic effect, hydrogen bonds, and salt bridges hold the four polypeptide chains together. Saturation of Haemoglobin With OxygenOxyhaemoglobin, the kind of haemoglobin that is saturated with oxygen molecules, and desaturated haemoglobin, the type of haemoglobin, respectively (deoxyhaemoglobin). OxyhemoglobinAs oxygen attaches to the heme portion of the haemoglobin molecule in red blood cells, oxyhemoglobin is created during physiological respiration. The pulmonary capillaries close to the lungs' alveoli are where this process takes place. The oxygen then travels via the bloodstream to cells where it is delivered and used as a terminal acceptor of electrons in the oxidative phosphorylation procedure that results in the synthesis of ATP. Ventilation, or breathing, may correct this state by removing carbon dioxide, which results in a shift up in pH, but it does not assist to combat a reduction in blood pH. There are two different types of haemoglobin: taut (tense) form (T) and relaxed form (R). The taut form, which has low oxygen affinity and releases oxygen in the tissues, is favored by a number of conditions, including low pH, high CO2, and high 2,3 BPG at the level of the tissues. The relaxed form, on the other hand, is favored by a high pH, a low CO2 level, or a low 2,3 BPG level, and it is more able to bind oxygen. The partial pressure of the system also impacts O2 affinity; with high partial pressures of oxygen (such as those found in the alveoli), the relaxed (high affinity, R) state is favored. The (low affinity, T) tense state, on the other hand, is preferred at low partial pressures (such as those seen in respiring tissues). A minor conformational change in the iron is also brought about by the binding of oxygen to the iron(II) heme, which pushes the iron toward the plane of the porphyrin ring. The change makes the three remaining heme units in haemoglobin more receptive to the binding of oxygen (thus, oxygen binding is cooperative). Haemoglobin DeoxygenationHaemoglobin that has not been bound with oxygen is known as deoxygenated haemoglobin (deoxyhaemoglobin). Deoxyhaemoglobin has a different absorption spectrum than oxyhaemoglobin. Compared to deoxyhaemoglobin, oxyhaemoglobin absorbs light at a substantially lower wavelength (660 nm) while somewhat increasing at 940 nm. Using this difference, a device known as a pulse oximeter may calculate how much oxygen is present in a patient's blood. This variation also explains the appearance of cyanosis, the blue to purple tint that tissues take on when hypoxia sets in. Haemoglobin that has lost its oxygen is paramagnetic, which means that magnetic fields very faintly attract it. A small magnetic field repels oxygenated haemoglobin, in contrast, due to a property known as diamagnetism. Vertebrate Haemoglobin DevelopmentMyoglobin and haemoglobin were split off from one another when lampreys diverged from jawed vertebrates, according to scientists. This separation of the two molecules allowed for the various activities of the two molecules to emerge and develop; myoglobin is more involved in oxygen storage while haemoglobin is responsible for oxygen transport. The genes for - and -like globins code for the constituent subunits of the protein. At 450?"500 million years ago, the gnathostome common ancestor, which descended from jawless fish, was also followed by a duplication event that gave rise to the genes that preceded these ones. Ancestral reconstruction studies indicate that the duplication ancestor of the ? and ? genes was a dimer formed of identical globin subunits, which subsequently developed to assemble into a tetrameric architecture following the duplication. The evolution of the genes allowed the possibility for haemoglobin to be constituted of several different subunits, a physical composition crucial to haemoglobin's capacity to transport oxygen. Haemoglobin capacity for both cooperative oxygen binding and allosteric regulation is influenced by the presence of numerous subunits. Later, the globin gene experienced a duplication event to generate the HBA1 and HBA2 genes. [These additional mutations and schisms have produced a varied spectrum of globin- ?- and ?- globin-like genes that are controlled such that certain forms arise at particular phases of development. The majority of Channichthyidae ice fish have lost the ability to produce haemoglobin as a result of adjusting to the cold. What are Haemoglobinopathies?Blood illnesses called haemoglobin disorders (or hemoglobinopathies) are passed down via families. The chemical known as haemoglobin, which is found within your red blood cells, transports oxygen throughout your body. A haemoglobin problem develops when the right amount of haemoglobin is not produced because it cannot perform its function. They comprise:
The signs and symptoms of haemoglobin abnormalities may range from non-existent to fatal. Even within each illness, there are varying degrees of impact on various individuals. All haemoglobin abnormalities result in some degree of anemia, which may leave you feeling depleted and exhausted and add additional stress to your heart as it works harder to circulate oxygen throughout your body. With sickle cell disease, there may also be excruciating blood vessel obstructions that may result in infections, organ damage, and other life-threatening problems. The following is a list of some common disorders that may develop when haemoglobin levels rise or fall:
Most Common Haemoglobin Characteristics in Mississippi
When Should A Person Consider Being Tested?Without a blood test, you won't be able to tell whether you have the sickle cell trait. In the best-case scenario, if you and your partner are unsure about your status, you should get a blood test before deciding to have a family. There is a possibility that you might transfer the faulty gene to your child if you have been diagnosed with sickle cell trait by a medical professional.
Next TopicHooke's Law Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










