Compound DefinitionIntroductionA compound is an ingredient or group of elements that has been chemically mixed in a certain ratio to form a material. Compounds, as opposed to mixtures, are made up of certain constituent parts and have unique characteristics that set them apart from their component parts. When atoms from various elements come together to form molecules or crystal lattices, a chemical reaction takes place, and that's how compounds are created. The atoms can join together in a variety of ways, including by exchanging electrons (covalent bonding), transferring electrons (ionic bonding), or establishing a metallic link. 
Chemical formulas are used to represent compounds and they contain information on the sorts and quantity of atoms that make up the compound. Water, for instance, has the chemical formula H2O, which denotes that it has two hydrogen atoms and one oxygen atom in it. Glucose, table salt, and carbon dioxide are some further prevalent examples of compounds (C6H12O6). Chemistry includes the study of compounds since it helps to understand the characteristics and behaviour of matter. In order to create new materials with specific qualities, create new drugs, and comprehend the chemical processes that take place in living things, chemists employ their knowledge of compounds. Types of CompoundsIn chemistry, there are various categories of compounds, each having its own specialities and traits. The different compounds can be grouped according to their composition, structure, and bonding among other criteria. Here, we will look at the types of compounds and their attributes. 1. Organic CompoundsOrganic substances are made up of carbon, hydrogen, and additional elements like oxygen, nitrogen, sulphur, and halogens. Proteins, carbohydrates, lipids, oils, polymers, and medications are only a few examples of the enormous range of natural and manufactured materials that contain them. The atoms or groups of atoms that give a molecule its distinctive qualities are known as functional groups, and they can be used to divide organic compounds into various groups. Alcohols are an illustration of an organic compound that has a hydroxyl group (-OH) linked to a carbon atom. Carboxylic acids, amines, and ketones have other forms of functional groups. 2. Inorganic CompoundsCompounds without carbon-hydrogen bonds are known as inorganic substances such as metals, salts, and minerals. For numerous biological processes as well as a variety of commercial operations, inorganic chemicals are crucial. Based on their composition and characteristics, inorganic substances can be divided into numerous classes. For instance, inorganic chemicals called bases can take hydrogen ions while inorganic compounds called acids can give hydrogen ions (H+) in solution. 3. Ionic CompoundsThe exchange of electrons between atoms results in the formation of ionic substances. They consist of cations, which are positively charged ions, and anions, which are negatively charged ions, held together by electrostatic forces. At room temperature, ionic compounds are typically solids with high melting and boiling temperatures. Ionic substances include calcium carbonate (CaCO3), magnesium oxide, and table salt (NaCl). In addition to being utilised to make ceramics, glass, and other materials, ionic compounds are crucial to numerous biological processes. 4. Covalent CompoundsCovalent compounds are created when atoms share electrons. They are composed of molecules joined by covalent bonds. Polar and nonpolar covalent molecules fall within these two types. Due to the unequal distribution of electrons, polar covalent compounds have an uneven charge distribution throughout the molecule. Water (H2O) and ammonia (NH3) are two examples of polar covalent molecules. Due to the uniform distribution of electrons across the molecule, nonpolar covalent compounds have a consistent distribution of charge. Methane (CH4) and oxygen gas (O2) are two examples of nonpolar covalent molecules 5. Metallic CompoundsMetal atoms make up substances known as metallic compounds. Metal-to-metal sharing of electrons results in the formation of metallic bonds, which hold them together. The malleability and strong electrical conductivity of metallic compounds are distinctive characteristics. Iron, gold, and copper are some examples of metallic compounds. Electronics, building and jewellery making are just a few examples of the diverse uses for metallic compounds. 6. Molecular CompoundsCompounds consisting of molecules are known as molecular compounds. They might be polar or nonpolar and are connected by covalent connections. In contrast to ionic compounds, molecules are often gases or liquids at room temperature, and thus have lower melting and boiling temperatures. Carbon dioxide (CO2), methane (CH4), and nitrogen gas (N2) are examples of molecular molecules. The usage of molecular compounds is widespread in industry and is crucial to many biological processes. 7. Binary CompoundsA compound with two elements is referred to as a binary compound. These substances, which can be either metals or nonmetals, come together to create molecules that have a specific atom-to-molecule composition. Ionic and covalent are the two categories into which binary compounds can be divided. Electrostatic forces hold together ionic binary compounds, which are made up of a cation and an anion. Covalent bonds keep nonmetals in covalent binary compounds, which are composed of nonmetals. The study of chemistry is fundamentally based on binary molecules, which are crucial to many chemical reactions. 8. Acidic CompoundsCompounds that can donate hydrogen ions (H+) in solution are referred to as acidic chemicals. They are distinguished by their sour taste and their capacity to create hydrogen gas when they interact with metals. Acidic substances include, for instance, hydrochloric acid (HCl), sulfuric acid (H2SO4), and acetic acid (CH3COOH). 9. Basic CompoundsBasic substances are those that can take up hydrogen ions (H+) in a solution. They are distinguished by their bitter taste and capacity to counteract acids. Calcium hydroxide (Ca(OH)2), sodium hydroxide (NaOH), and ammonia (NH3) are examples of basic substances. 10. SaltsThe reaction between an acid and a base produces chemicals known as salts. Ionic bonds hold their composition of cations and anions together. The majority of salts have high melting and boiling temperatures and are often solid at normal temperatures. Calcium sulphate, potassium nitrate, and sodium chloride are a few examples of salts. 11. HydratesCompounds known as hydrates have crystal structures that include water molecules. They develop as a result of a water-ionic chemical reaction. By losing water molecules when heated, hydrates are distinguished from other substances. CuSO4•5H2O, which is a pentahydrate of copper sulphate, and MgSO4•7H2O, which is a heptahydrate of magnesium sulphate, are two examples of hydrates. 12. Coordination CompoundsCompounds known as coordination compounds have a metal ion at the centre surrounded by a collection of ligands. Covalent coordinate bonds are used to attach molecules or ions known as ligands to the metal ion. Color and magnetic behaviour are two unusual characteristics of coordination molecules. Iron- and magnesium-containing substances like haemoglobin and chlorophyll are examples of coordination compounds. Examples of CompoundsVarious elements such as H2O (water), CO2, or O2, or can be of the same element, such as in the case of O2 (oxygen) or N2 (nitrogen). Compound examples include the following: 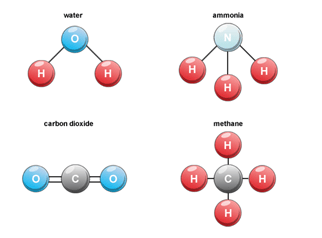
Sodium Chloride (NaCl) - This is a common compound that is also known as table salt. It is made up of sodium (Na) and chlorine (Cl) atoms that are chemically bonded together. Sodium chloride is used in many different applications, including food seasoning, water treatment, and chemical manufacturing. Carbon Dioxide (CO2) - This is a compound that is made up of one carbon (C) atom and two oxygen (O) atoms. Carbon dioxide is a greenhouse gas that is emitted by many different sources, including fossil fuel combustion, deforestation, and cement production. It is also used in many different industrial processes, such as dry ice production and fire extinguishers. Water (H2O) - This is a compound that is made up of two hydrogen (H) atoms and one oxygen (O) atom. Water is essential for life on Earth and is used in many different applications, including agriculture, manufacturing, and energy production. Methane (CH4) - This is a compound that is made up of one carbon (C) atom and four hydrogen (H) atoms. Methane is the primary component of natural gas and is also produced by many different sources, including livestock and landfills. It is used as a fuel in many different applications, including heating and transportation. Ethanol (C2H5OH) - This is a compound that is made up of two carbon (C) atoms, six hydrogen (H) atoms, and one oxygen (O) atom. Ethanol is a type of alcohol that is produced by fermentation and is used as a fuel, solvent, and antiseptic. It is also used in the production of many different products, including cosmetics, pharmaceuticals, and plastics. Ammonia (NH3) - This is a compound that is made up of one nitrogen (N) atom and three hydrogen (H) atoms. Ammonia is used in many different applications, including fertilizer production, refrigeration, and cleaning products.
Next TopicControl Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










