Neutron DefinitionThe neutron is a subatomic particle with the symbol n or n0 that is slightly heavier than a proton and has a neutral charge (one that is neither positive nor negative). Atomic nuclei consist of protons and neutrons. Protons and neutrons are referred to as "nucleons" because of how similar they function within the nucleus and because they have masses roughly equivalent to one atomic mass unit. Nuclear physics explains their interactions and characteristics. Each proton and neutron is made up of three quarks; hence they are not fundamental particles. The arrangement of electrons in orbit around an atom's heavy nucleus is a major factor in determining its chemical characteristics. The charge of the nucleus, which is determined by the number of protons, or atomic number, determines the electron configuration. The neutron number is the total amount of neutrons. Although neutrons have no impact on electron configuration, the mass of the nucleus is equal to the sum of its atomic and neutron numbers. Isotopes are variations of an element's chemical atom that only vary in neutron number. For example, the atomic number six element carbon has two isotopes: the common carbon-12, which has six neutrons, and the uncommon carbon-13, which has seven neutrons. Fluorine is one of the few elements that only have one stable isotope in nature. Tin has ten stable isotopes. Some elements, like technetium, don't have any stable isotopes at all. 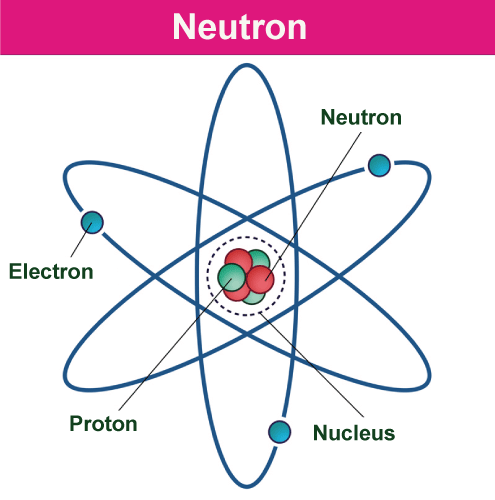
Both the atomic and neutron numbers affect the characteristics of an atomic nucleus. The long-range electromagnetic force repels protons because of their positive charge, while the nuclear force, which is considerably stronger yet short-range, bonds nucleons tightly together. Except for the hydrogen nucleus, which has a single proton, neutrons are necessary for the stability of nuclei. Nuclear fission and fusion generate an immense number of neutrons. They play a major role in the fission, fusion, and neutron capture processes that lead to the nucleosynthesis of chemical elements inside stars. The creation of nuclear energy requires neutrons. James Chadwick discovered the neutron in 1932, and throughout the next ten years, neutrons were utilized to induce a wide variety of nuclear transmutations. When nuclear fission was discovered in 1938, it wasn't long before it was understood that if a fission event generated neutrons, each of these neutrons may then trigger more fission events in a chain reaction known as a nuclear chain reaction. The first self-sustaining nuclear reactor (Chicago Pile-1, 1942) and the first nuclear weapon (Trinity, 1945) were the results of these developments. Neutron generators, research reactors, and spallation sources are reliable neutron sources that generate free neutrons for use in neutron scattering and irradiation investigations. With a mean lifespan of around 15 minutes, a free neutron spontaneously decays into a proton, an electron, and an antineutrino. Free neutrons may be a biological threat depending on dosage, even though they don't directly ionize atoms. Instead, they indirectly produce ionizing radiation. The inherent radioactivity of spontaneously fissionable materials in the Earth's crust and cosmic ray showers both contribute to the tiny "neutron background" flux of free neutrons that occurs on Earth. Discovery of NeutronsThe Rutherford Nuclear Model of Atom (Alpha particle scattering experiment) discovered its existence. In this experiment, when the majority of the alpha particles pass without deviating, some travel through lesser angles, and others pass through angles more than 180 degrees; it also provides us with extraordinary information about the organization of the component particles. It indicated the presence of a mass particle and pointed to the existence of a particle in the center of the atom (the nucleus). James Chadwick subsequently discovered the neutron in 1932. The model would have issues since electrons have very little mass and atoms only have proton mass. As a result, it was determined that the atom must include a neutral particle of a proton's mass or more. Rutherford understood that without the existence of another particle, the atomic masses of various elements could not be explained. In 1920, Rutherford claimed that there was a specific kind of neutral particle with a mass equal to that of a proton. James Chadwick found a particle in 1932 while researching the artificial transformation of atoms, which Rutherford had predicted in 1920. When swiftly traveling alpha particles strike a thin foil of beryllium, the material transforms into carbon and releases a neutral particle with the same mass as the proton. The particle, which was discovered in several other processes, was given the name neutrons due to its neutral nature. Neutron's Properties
Neutron mass and charge
Detection of NeutronsNeutrons cannot be directly detected using the typical search method for an ionization trail, such as in a cloud chamber. Other neutron detection methods, such as enabling them to interact with atomic nuclei, are more often utilized. Neutrons that elastically scatter off atoms may produce an ionization track that can be detected. Thus, the widely used neutron detection techniques may be divided into groups based on the nuclear processes they employ, namely elastic scattering or neutron capture. Neutron Capture for Neutron DetectionThe energy released during neutron capture processes is often converted into electrical signals as a popular technique for neutron detection. The probability of absorbing a neutron is known as the neutron capture cross-section, and certain nuclides have a large cross-section. The compound nucleus generates more readily visible radiation after neutron capture, such as an alpha particle, which is subsequently picked up by detectors. For this, it is advisable to employ the nuclides 3He, 6Li, 10B, 233U, 235U, 237Np, and 239Pu. Elastic Scattering for the Detection of NeutronsThe nucleus that was hit may rebound as a result of the elastic scattering of neutrons off of it. A lighter nucleus, like hydrogen or helium, may receive more energy from a neutron kinematically than a heavier nucleus can. Fast neutron detectors are those that depend on elastic scattering. Collisions between reversing nuclei may ionize and excite more atoms. This produces charge and scintillation light, which may be collected to provide a detectable signal. Distinguishing these signals from false signals generated by gamma radiation in the same detector is a difficult task in rapid neutron detection. Although certain inorganic scintillator-based detectors have been created to selectively detect neutrons in mixed radiation fields intrinsically without any further procedures, techniques like pulse shape discrimination may be utilized to separate neutron signals from gamma-ray signals. Because they don't need a moderator, fast neutron detectors have the benefit of being able to measure the neutron's energy, arrival time, and, in some situations, incidence direction. Neutron CompoundsTetraneutrons and DineutronsTetraneutrons, or stable clusters of four neutrons, have been speculated by a group at the CNRS Laboratory for Nuclear Physics under the direction of Francisco-Miguel Marqués based on research into the nuclear fusion of beryllium-14. This is especially intriguing since, according to current theory, these clusters shouldn't be stable. Susumu Shimoura of the University of Tokyo in Japan and his colleagues stated that they had made the first experimental observation of the alleged tetraneutrons in February 2016. According to nuclear physicists from all around the globe, the finding would mark a significant advancement in the discipline and expand our knowledge of nuclear forces if it were to be verified. Another possible particle is the dineutron. The dineutron emission in the decay of 16Be was first noticed in 2012, according to research done by Artemis Spyrou of Michigan State University and colleagues. A small emission angle between the two neutrons serves as proof of the dineutron nature. Using interactions that are typical for this mass area, the scientists determined that the energy required to separate two neutrons was 1.35(10) MeV, which is in perfect accord with simulations made using a shell model. Neutronium and neutron starsIt is hypothesized that nucleons and electrons collide to form bulk neutronic matter, or neutronium, at very high pressures and temperatures. Neutron stars are thought to be where this occurs. A neutron star's intense pressure may cause the neutrons to take on a cubic symmetry, allowing for more compact packing. Applications of NeutronNumerous nuclear processes depend heavily on the neutron. For instance, neutron activation, which causes radioactivity, often follows neutron capture. The development of nuclear reactors and nuclear weapons, in particular, has benefited from our understanding of neutrons and their behavior. The neutron absorption of atoms like uranium-235 and plutonium-239 causes them to fission. Neutron scattering facilities often utilize cold, warm, and hot neutron radiation, which is used in a manner similar to how X-rays are used to analyze the condensed matter. In terms of atomic contrasts by distinct scattering cross sections, sensitivity to magnetism, energy range for inelastic neutron spectroscopy, and deep penetration into materials, neutrons are complimentary to the latter. Current research into neutron microscopy and neutron/gamma-ray tomography is being pushed by the creation of "neutron lenses" based on total internal reflection inside hollow glass capillary tubes or by reflection off dimpled aluminum plates. Neutrons are often used to stimulate and delay the gamma-ray emission of certain materials' constituent components. This serves as the foundation for both prompt gamma neutron activation analysis (PGNAA) and neutron activation analysis (NAA). While PGNAA is most often used to analyze industrial bulk materials on conveyor belts and underground rocks near boreholes, NAA is most frequently utilized to analyze tiny samples of materials in a nuclear reactor. The detection of light nuclei, particularly the hydrogen present in water molecules, is another use of neutron emitters. A significant portion of the energy of a fast neutron is lost upon collision with a light nucleus. A neutron probe may detect the amount of water in the soil by monitoring the speed at which slow neutrons bounce off hydrogen atoms and return to the probe. Fission and Fusion Neutrons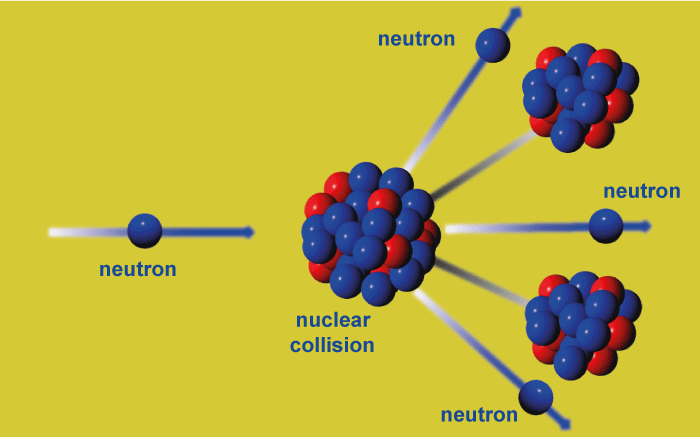
FissionFast neutrons are free neutrons that have kinetic energies of 1 MeV (1.6 1013 J), giving them a speed of around 14000 km/s (5% of the speed of light). To differentiate them from high-energy neutrons generated in cosmic showers or accelerators and lower-energy thermal neutrons, they are known as fission energy or fast neutrons. Nuclear reactions like nuclear fission generate fast neutrons. As previously mentioned, fission neutrons have kinetic energies ranging from 0 to 14 MeV, a mean energy of 2 MeV (for 235U fission neutrons), and a mode of only 0.75 MeV, which means that more than half of them do not qualify as fast, and therefore have almost no chance of initiating fission in fertile materials, such as 238U and 232Th. Moderation is the conversion of fast neutrons into thermal neutrons. With a neutron moderator, this is accomplished. Neutrons are often tempered in reactors using heavy water, light water, or graphite. FusionThe fusion reaction rate rises sharply with temperature until it reaches its maximum, after which it progressively declines. The D-T rate peaks at a greater value and a lower temperature (about 70 keV, or 800 million kelvins) than other processes often thought of as producing fusion energy. The fusion process that generates neutrons with 14.1 MeV of kinetic energy and moves at 17% of the speed of light is called D-T (deuterium-tritium) fusion. D-T fusion is also the fusion process that ignites the simplest, achieving near-peak rates even when the kinetic energy of the deuterium and tritium nuclei is a thousandth of the 14.1 MeV that will be created. Neutrons from other fusion processes are substantially less energetic. Half of the time, D-D fusion generates a 2.45 MeV neutron and helium-3; the other half generates tritium, a proton, and no neutron. No neutron is created by D-3He fusion.
Next TopicWildlife Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










