Redox Reaction DefinitionRedox reactions are chemical reactions that involve the transfer of electrons from one chemical species to another. The term redox comes from the combination of two words, reduction and oxidation. Reduction is the process of gaining electrons, whereas oxidation is the process of losing electrons. Redox reactions play an important role in many chemical and biological processes, including respiration, combustion, and corrosion. In this article, we will explore redox reactions in more detail. 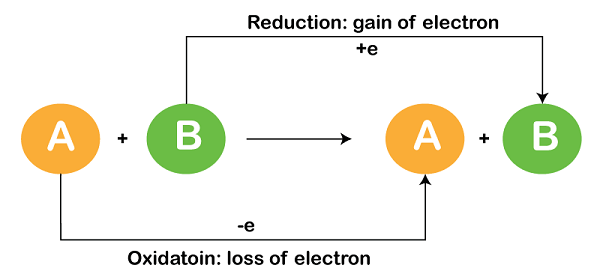
Redox ReactionsRedox reactions involve the transfer of electrons from one chemical species to another. The species that loses electrons is said to be oxidized, whereas the species that gains electrons is said to be reduced. For example, consider the following reaction: Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s) In this reaction, zinc (Zn) is oxidized to form zinc ions (Zn2+), and copper ions (Cu2+) are reduced to form copper (Cu). The oxidation and reduction reactions can be written separately as follows: Zn(s) → Zn2+(aq) + 2e- Cu2+(aq) + 2e- → Cu(s) The electrons that are lost by zinc atoms are gained by copper ions, which reduces them to copper atoms. This transfer of electrons is what makes this a redox reaction. Oxidation NumbersOxidation numbers are used to keep track of electrons in redox reactions. The oxidation number of an atom in a molecule or ion is the charge that atom would have if the electrons were completely transferred to the most electronegative atom. For example, the oxidation number of hydrogen in H2O is +1, and the oxidation number of oxygen is -2. The sum of the oxidation numbers of all the atoms in a molecule or ion must be equal to the charge on the molecule or ion. Oxidizing and Reducing AgentsAn oxidizing agent is a chemical species that causes oxidation by accepting electrons from another species. In the above example, Cu2+ is the oxidizing agent. A reducing agent is a chemical species that causes reduction by donating electrons to another species. In the above example, Zn is the reducing agent. The oxidizing agent is reduced, whereas the reducing agent is oxidized. In other words, the reducing agent loses electrons, and the oxidizing agent gains electrons. Half-reactionsRedox reactions can be broken down into half-reactions, which show the oxidation and reduction processes separately. Each half-reaction shows the species that is being oxidized or reduced, as well as the electrons that are being gained or lost. For example, the half-reactions for the above example are: Zn → Zn2+ + 2e- (oxidation) Cu2+ + 2e- → Cu (reduction) In the first half-reaction, zinc atoms are being oxidized to form zinc ions, and two electrons are being lost. In the second half-reaction, copper ions are being reduced to form copper atoms, and two electrons are being gained. Balancing Redox ReactionsBalancing redox reactions involves balancing the number of electrons transferred as well as the number of atoms in the reactants and products. One method for balancing redox reactions is the half-reaction method, which involves balancing the oxidation and reduction half-reactions separately. Another method is the oxidation number method, which involves determining the changes in oxidation numbers for each element and using that information to balance the reaction. Applications of Redox ReactionsRedox reactions play an important role in many chemical and biological processes. Some examples include:
Redox reactions also play a critical role in the environment. For instance, many natural processes, such as the cycling of carbon, nitrogen, and sulfur, involve redox reactions. These reactions are important for the growth and survival of plants and other organisms. Additionally, redox reactions play a key role in the breakdown of pollutants in the environment. For example, microorganisms can use redox reactions to break down organic contaminants in soil and groundwater. Furthermore, redox reactions have important industrial applications. One example is the production of chemicals such as chlorine and sodium hydroxide through the electrolysis of brine (a solution of sodium chloride in water). This process involves a redox reaction in which chloride ions are oxidized to chlorine gas at the anode, while water is reduced to hydrogen gas at the cathode. Another example is the use of redox reactions in the production of metals such as aluminum and titanium, which are produced through the reduction of their respective oxides using a reducing agent such as carbon. Redox reactions are also important in analytical chemistry. Redox titrations are a class of analytical methods that use redox reactions to determine the concentration of a species in a sample. One example is the determination of the iron content in a sample using a redox titration with potassium permanganate. In this titration, iron is oxidized to Fe3+ by the permanganate ion, which is reduced to Mn2+. The point at which all of the iron has been oxidized can be detected by a change in color of the solution, which is due to the disappearance of the permanganate ion. ConclusionRedox reactions are an important type of chemical reaction that involve the transfer of electrons from one chemical species to another. These reactions play an important role in many chemical and biological processes, including respiration, combustion, corrosion, and batteries. Understanding redox reactions is important for many applications, including energy production, corrosion prevention, and battery design. The half-reaction and oxidation number methods can be used to balance redox reactions, and the concepts of oxidizing and reducing agents, oxidation numbers, and half-reactions are important for understanding redox reactions.
Next TopicRock Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










