Enantiomers DefinitionThe notion of enantiomers is related to the Molecule module and is included in the subject matter of Chemistry. It uses formulae to discuss the composite components. Molecules that have identical interconnectivity and geometrical structure are said to be stereoisomers. One of the two categories of stereoisomers is the enantiomer. The first category includes diastereomers. They are compounds that are entirely symmetrical and not mirrored. There are molecules that resemble one another but are not perfect replicas of one another, much like a pair of twins with distinguishing traits. These substances are referred to as enantiomers. Understand enantiomers' chemistry, chiral carbon's significance, and enantiomers' physical and chemical characteristics. What is Stereochemistry?The study of the various spatial configurations of atoms in molecules is the focus of the field of chemistry known as stereochemistry. A brief introduction to history is usually necessary before discussing a particular area of science and technology, such as stereochemistry. Stereochemistry, sometimes known as the "chemistry of space," is the study of how atoms and groups are arranged spatially inside molecules. The discovery that tartaric acid salts from a wine production vessel can rotate plane-polarized light, as compared with tartaric acid salts from other sources, was made by French chemist Louis Pasteur in 1842, which is when stereochemistry first emerged. Optical isomerism provides a theoretical explanation for this phenomenon.
Different Stereoisomer Types
The term "stereoisomerism" describes the isomerism that results from different spatial configurations of an atom's functional groups or atoms themselves. Although the atoms in these kinds of isomers are arranged differently geometrically, their constitutions are the same. Enantiomers and diastereomers may be used as a general classification for the two forms of stereoisomers. What are Enantiomers?Enantiomers are the names given to the chemical pairings. Enantiomers are chemical pairings that cannot be superimposed as mirror copies of one another. Enantiomers may be thought of as two pairs of hands when compared to other objects. Let's use the example of Steven and Kevin, identical twins who are right- and left-handed, respectively. One day, you mistakenly believe you are staring at Steven when you cross paths with one of them in class. Not at all, no. Since Kevin is writing with his left hand, this is him. Similar to how Steven and Kevin are identical twins, there are certain pairings of substances or molecules that we mistakenly believe to be the same thing because they are mirror reflections of one another However, there are differences between the two. Enantiomers have identical chemical structures in all other respects. The term "optical isomers" describes a pair of enantiomers, either dextro (d or +) or levo (l or -), that are distinguished from each other through the angle in which polarised light spins when it breaks down in solution. When two distinct enantiomers are present in equal quantities, a racemic mixture is produced, which cannot spin light that is polarised as the refractive index of a single enantiomer has been cancelled out by the other. 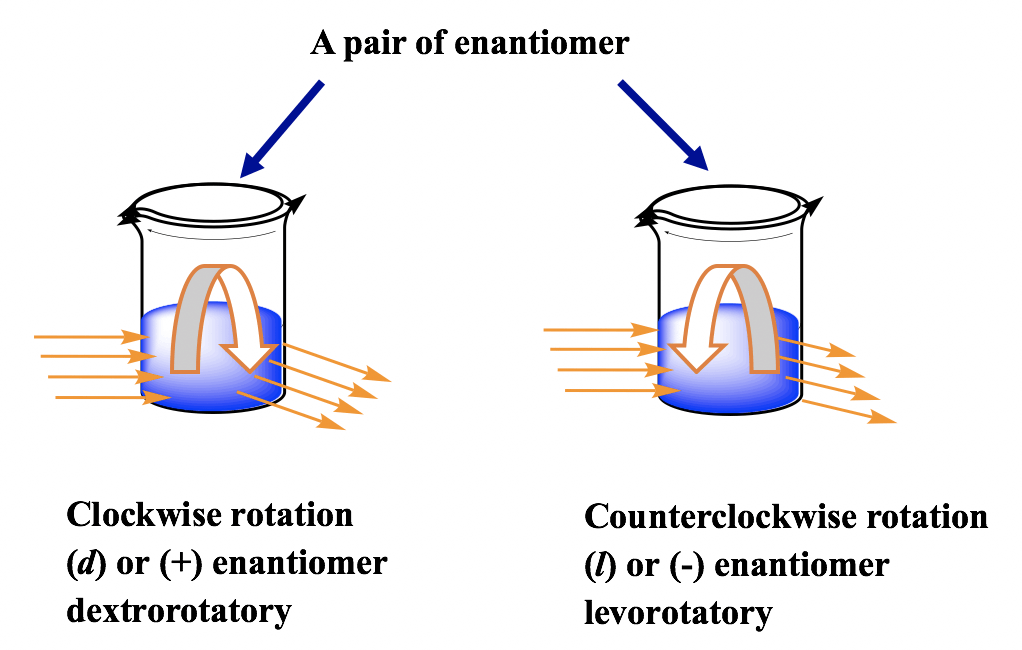
As they cannot be superimposed, no matter how you spin them, we can also see the molecule being superimposed on the left and right. Enantiomers are consequently formed from the two molecules. After discussing the concept of an enantiomer, let's analyze its chirality and other characteristics:
Enantiomer Structure
Enantiomer Properties
Enantiomer Chemistry and TopologyIt is well known that chemical substances that display stereoisomerism and have various enantiomeric structures typically interact with other enantiomeric molecules. Enantiomers are, at this time, thought to be specific biological compounds. In addition, it's essential to keep in mind that two separate enantiomers of an identical molecule might have entirely different effects on a variety of species. Commonly, this phenomenon is seen in how different medications affect people. In certain circumstances, the required physiological advantages could only be achieved by one of a drug's enantiomers. 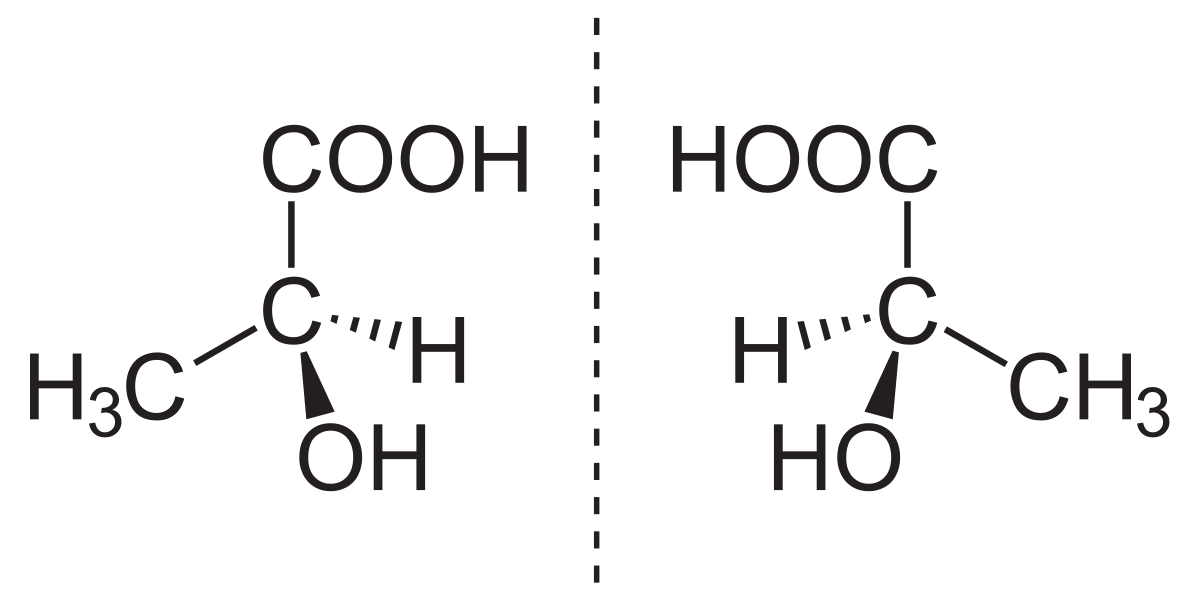
The molecular characteristics of a pair of enantiomers are the same, but when they interact with other chiral compounds, they change. Consider a handshake between two individuals who both shake with their right hands. The move would feel extremely different, however, if one person shook hands with the other using her right hand and the other using his left. Similar to this, the chemical reactions that occur when two enantiomers interact with other chiral substances will be different. Enantiopure PharmaceuticalsPharmaceutical companies may now profitably commercialize the individual enantiomers of medications that were previously only available as a racemic combination because of developments in industrial chemical processes. In order to improve therapeutic effectiveness, this marketing approach of chiral-specific drugs from racemic drugs that are currently licensed and available is often used. A chiral switch occurs when a medication is changed from a racemic form to an enantiopure form, and the process is also known as chiral switching. The effects of the enantiomers may vary in various circumstances. Propoxyphene is a particularly intriguing instance. Eli Lilly and Company sells the enantiomeric pair of propoxyphene separately. Levopropoxyphene, a strong antitussive sold under the brand name Novrad, and dextropropoxyphene, an analgesic drug sold under the brand name Darvon, are the other partners. It's notable to point out that DARVON and NOVRAD, the brand names for the medications, both allude to their similar chemical makeup. There could not be any clinical advantage for the patient in other situations. Single-enantiomer medications are independent from the racemic combination in various legal systems and are patented. One enantiomer could be the sole active one. Alternately, it's possible that both components be functional; in this instance, separating the combination has no real advantages other than extending the drug's patent.
Next TopicEquator Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share