Electrostatic Force DefinitionThe electrostatic force is defined as the tendency of two charged particles to attract or repel one another. The Coulomb interaction or Coulomb force are other names for it. For instance, the electrostatic force between an atom's protons and electrons is what keeps it stable. An ionic molecule is bound by the electrostatic bonding force, which is significant in chemistry. 
MeaningThe study of static electricity, electric charges that are at rest, is done by the science of electrostatics. Some materials, like amber, have been known to capture light particles after rubbing since classical times. The word "electricity" thus derives from the Greek term for amber. Electric charges exert forces on one another, which results in electrostatic phenomena. Coulomb's law can be used to characterize these forces. Even while electrostatically induced forces seem to be relatively weak, some of them are actually rather powerful. The gravitational force between two objects is about 36 orders of magnitude, weaker than the force between an electron as well as a proton, which combined make up a hydrogen atom. Instances of electrostatic phenomena range from the simplest, like the attraction that draws plastic wrap to one's hands after it has been separated from a package to the apparently natural bursting of grain silos, damage caused by electronic parts between manufacturing, and the operation of photocopiers and laser printers. When an object comes into contact with another surface, charge builds up on its surface, which is known as electrostatics. The effects of charge exchange are typically only noticed when at least one of the elements has a high resistance to electrical flow because the electrical charges that transfer remain trapped there for an adequate amount of time for their effects to be noticed. Charge exchange occurs regardless of whether any two surfaces contact and separate. Then, until they eventually bleed down to the ground or are swiftly neutralized by a discharge, these charges remain on the object. The common occurrence known as a static "shock" is brought on by the neutralization of charge that has accumulated in the body due to interaction with insulated surfaces. Coulomb's LawAccording to Coulomb's law, "The electrostatic force, or the force of repulsion or attraction among two point charges, is directly & inversely proportional to the product of the values of the charges and the square of their separation." The following is what this law says regarding the force between two separate particles:
Consider bringing the two charged elements together. If the charges are opposed, or if one charge is positive while the other is negative, there will definitely be an attraction. If both the charges are negative or positive, however, they will repel one another. In other words, similar charges repel while opposing charges attract. The ability of a particle or object to draw in or repel other kinds of particles or things due to its electric charge is known as the Coulomb force, sometimes referred to as electrostatic force and the Coulomb link. The electric force was named after Charles-Augustin de Coulomb, a French physicist who published the results of an experimental investigation into the appropriate mathematical description of this force in 1785. One of the basic forces in physical reality is the electric force. They are connected by a straight line of force that is exerted. The electrostatic force among two charges is repellent if their signs are the same and attracting if their signs differ. Coulomb MeaningThe International System of Units (ISO) standard unit of electric charge is the coulomb (C). The amount of power a 1-ampere (A) current can carry in a second is known as the flux. 1 C is roughly equivalent to 6.24 x 1018 protons or electrons in terms of electrical charge. 6.24 quintillion particles are involved in this. According to the SI standard, the 'coulomb' is a derived unit, meaning it is created using any of the 7 basic units, in this case, the ampere and the second. Prior to 2018, the derivative units were built from the base units, which were the basis for the SI standard. The standard is now based on seven defining constants, from which all base & derived units can be formed. Nonetheless, the base & derived units have been kept in the SI standard due to how well-established they are. HistoryAncient societies in the Mediterranean region were aware that some things, like amber rods, could be attracted to feathers as well as pieces of paper by rubbing them with cat fur. When Thales of Miletus observed that friction might make an amber object magnetic, he gave the earliest known description of static electricity about 600 BC. 
William Gilbert, an English scientist, conducted a thorough investigation into electricity and magnetism around 1600. He distinguished between the lodestone effect and static electricity created by rubbing amber. In order to describe the ability of amber to attract small items after being rubbed, he created the New Latin term electricus, which means "of amber" or "like amber." The English words "electric" & "electricity" originated from this relationship and were first used in literature in Thomas Browne's Pseudo Doxia Epidemica in 1646. Franz Aepinus, who proposed the inverse-square law in 1758, and Daniel Bernoulli, who measured the force among capacitor plates in the 18th century, all thought that the electrical force reduced with distance in a manner similar to how the force of gravitation did. Based on studies with electrically charged spheres, Joseph Priestley of England became one of the first to propose that the force of electricity followed an inverse-square law, similar to Newton's law of universal gravity. He didn't, however, make any generalizations or add to this. He proposed in 1767 that the square of the inverse of the distance between them would determine how much force there was between charges. Electrostatic Force CharacteristicsHere are some informational details about the electrostatic force's traits:
Examples of Electrostatic Force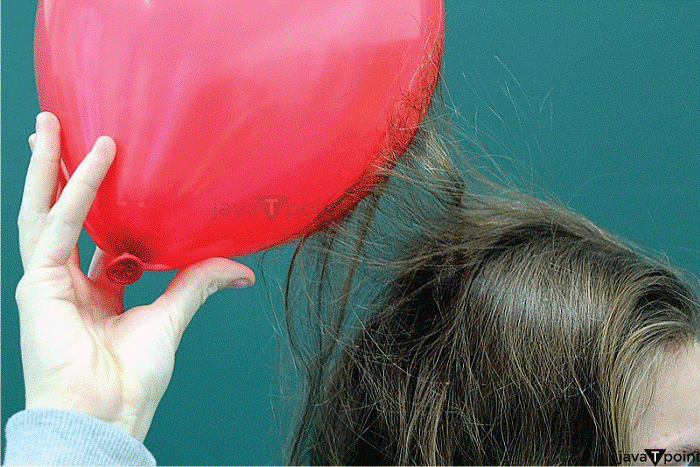
Examples of electrostatic forces are as follows:
Electrostatic Force ApplicationsHere are a few general examples of how the electrostatic force is used: 1. Van de Graaff GeneratorVan de Graaff generators, also known as Van de Graaffs, are utilized for serious study as well as stunning demonstrations of high voltage caused by static electricity. The first was created by "Robert Van de Graaff" in 1931, for application in nuclear physics research. Van de Graaff uses rough & pointed surfaces, conductors, & insulators to generate large static charges and, as a result, large voltages. It flows swiftly to the spheres outside, allowing for the deposition of an extremely large excess charge. Practical restrictions are necessary because strong electric fields polarise and ultimately ionize the materials they surround, generating free charges that either neutralize excess energy or let it escape. However, 15 million volt voltages are well within the range of practical application. 2. XerographyXerography, an electrostatic process, is what most copy machines do. A positive charge is shot onto a selenium-coated aluminium drum from spots on a corotron. A fascinating characteristic of selenium is that it functions as a photoconductor. In other words, while it's dark, selenium acts as an insulator, and when it's exposed to light, it acts as a conductor. 3. Inkjet PrintersLaser printers utilize the xerographic method to create images of superior quality on paper by using a laser to create an imprint on the photoconductive drum. The laser printer takes the output from the computer in its most common use, and it can produce high-quality output due to the accuracy with which the laser light is capable of being controlled. Many laser printers process a lot of information, such as creating intricate letters or fonts, as well as, in the past, might have had a computer inside that was more powerful compared to the one sending the raw data for printing.
Next TopicHomophones Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










