Beer-Lambert Law DefinitionThe Beer-Lambert Law, also known as the Beer-Lambert-Bouguer Law, is a fundamental principle of spectroscopy that relates the absorbance of light by a substance to its concentration in a solution. Numerous disciplines, including chemistry, biochemistry, environmental science, and medicine, are affected by this law. In this article, we will discuss the Beer-Lambert Law definition, its applications, and how it can be used to determine the concentration of a solution. The Beer-Lambert Law Explained.The connection between the amount of light absorbed by a solution and the concentration of the absorbing species is described by the Beer-Lambert Law, a mathematical formula. The rule is predicated on the idea that a solution's absorbance is inversely correlated with the concentration of the absorbing species and the length of the light's passage through the solution. This can be expressed mathematically as follows: A = εlc where A is the absorbance of the solution, ε is the molar extinction coefficient or absorptivity of the absorbing species, c is the concentration of the absorbing species, and l is the path length of the light through the solution. The Beer-Lambert Law is a linear relationship that holds true for dilute solutions where the concentration of the absorbing species is less than 10^-3 M. In more concentrated solutions, the relationship between absorbance and concentration becomes nonlinear due to factors such as self-absorption and intermolecular interactions. 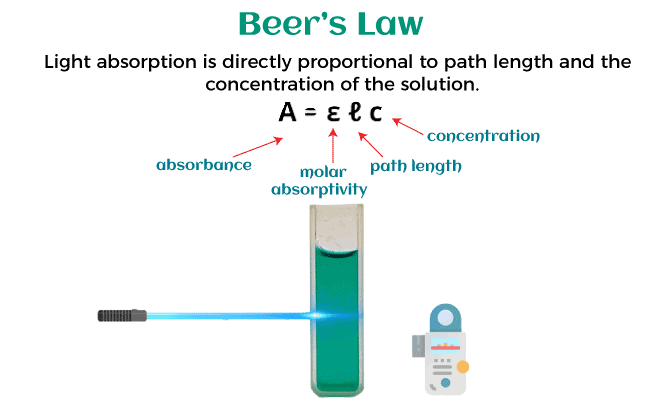
Uses for the Beer-Lambert LawThere are multiple uses for the Beer-Lambert Law across numerous industries. Below is a discussion of some of the Beer-Lambert Law's most typical uses.
Derivation of the Beer-Lambert LawThe Beer-Lambert Law can be derived from the first principles using the principles of electromagnetic radiation and quantum mechanics. The derivation is beyond the scope of this article, but we will provide a brief overview of the key concepts involved. The Beer-Lambert Law is based on the fact that when light passes through a solution, it is absorbed by the molecules in the solution. The concentration of the light-absorbing species and the light's route through the solution both affect how much light is absorbed. This relationship can be expressed mathematically as follows: dI = -αIdx Where dI is the change in intensity of the light as it passes through a small distance dx, I is the initial intensity of the light, and α is the absorption coefficient of the solution. Using the principle of mass action, we can relate the absorption coefficient α to the concentration of the absorbing species and the molar extinction coefficient ε: α = εc Substituting this expression into the equation for dI, we obtain: dI = -εcx dx Integrating both sides of the equation from 0 to l (the path length of the light through the solution), we obtain: I/I0 = e^(-εcl) Where I0 is the initial intensity of the light, and I is the intensity of the light after it has passed through the solution. This equation is known as the Beer-Lambert Law. How Important Concentration IsHow many molecules it interacts with will determine how much of the light is absorbed. Let's say you have an organic dye that is really brightly colored. It will have a relatively high absorption if it is in a moderately concentrated solution since there are several molecules interacting with the light. However, it could be quite challenging to detect any color at all in a solution that is extremely diluted. The absorption will be quite low. Let's say you wished to compare this color to another substance. You couldn't compare them logically to see which one absorbed lighter unless you were careful to account for the concentration. Significance of Container FormThis time, imagine that you had an extremely diluted dye solution in a cube-shaped container so that the light could pass through it in 1 cm. It's unlikely that the absorbance will be particularly high. On the other hand, imagine if the light was directed via a 100-cm-long tube filled with the same solution. Since more molecules are involved in the interaction, more light would be absorbed. Once more, you must take into account the length of the solution the light is going through if you wish to make meaningful comparisons across solutions. The Beer-Lambert Law takes concentration and solution duration into account. Molecular AbsorptivityYou may rewrite the Beer-Lambert rule to get the following expression for (the molar absorptivity): ϵ=Alc Keep in mind that a solution's absorbance will change depending on the concentration or the size of the container. By dividing by both the concentration and the length of the solution that the light travels through, molar absorptivity makes up for this. In essence, it calculates a number for what the absorbance would be given a typical set of circumstances?light passing through a solution of 1 mol dm-3 at a distance of 1 cm. As a result, you may compare one molecule to another without having to worry about solution concentration or duration. Molar absorptivity values can vary greatly. For instance, the UV-visible spectrum of ethanal has two absorption peaks, both in the ultra-violet. Both of these correspond to the promotion of an electron from an oxygen lone pair into a pi anti-bonding orbital and from an anti-bonding orbital into a bonding orbital. Take note that absorptivity is not expressed in units. Since the units are mol-1 dm3 cm-1 and the length is assumed to be in cm, it is a very typical assumption. Limits of the Beer-Lambert EquationWhile the Beer-Lambert Law is a powerful tool for measuring the concentration of a solution, it does have some limitations. Some of the most important limitations are discussed below. 1. Nonlinear Concentration DependenceAs mentioned earlier, the Beer-Lambert Law is only valid for dilute solutions where the concentration of the absorbing species is less than 10^-3 M. In more concentrated solutions, the relationship between absorbance and concentration becomes nonlinear due to factors such as self-absorption and intermolecular interactions. 2. Spectral InterferencesThe Beer-Lambert Law assumes that the absorbance of a solution is due to a single absorbing species. However, in many cases, the absorbance may be due to multiple species that have overlapping absorption spectra. This can lead to errors in concentration measurements. 3. Instrumental LimitationsThe accuracy of concentration measurements using the Beer-Lambert Law depends on the accuracy of the spectrophotometer used to measure absorbance. Instrumental limitations such as stray light, detector noise, and instrument drift can all affect the accuracy of concentration measurements. Beer-Lambert Law and BeerThe Beer-Lambert Law is also relevant to the brewing of beer, as it can be used to determine the concentration of various compounds in the beer. The most important application of the Beer-Lambert Law in brewing is in determining the bitterness of the beer. The bitterness of beer is due to the presence of iso-alpha acids, which are formed by the isomerization of alpha acids during the boiling of the wort. The concentration of iso-alpha acids can be measured using UV-Vis spectroscopy and the Beer-Lambert Law. The Beer-Lambert Law can also be used to determine the concentration of other compounds in beer, such as melanoidins and polyphenols. Graph of Beer Lambert's LawThe Molar Extinction Coefficient () value is calculated using a graphical technique. Plotting the values of absorbance (on the y-axis) against various concentration values (on the x-axis) and calculating the slope of the line yields this number. It is noted that a straight line is obtained, going through the origin. Having said that, A=εbc Where, A: The solution's absorption c: The solution's concentration ε: Molecular extinction coefficient b: The medium's thickness As a result, b is the graph's slope. Finding the value of is easy since we know the value of b. 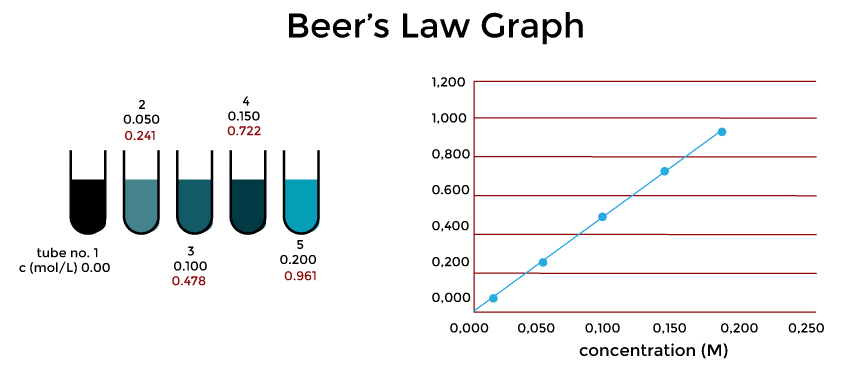
ConclusionIn conclusion, the Beer-Lambert Law is a fundamental spectroscopic concept that connects a substance's concentration in a solution to how much light it can absorb. Numerous disciplines, including chemistry, biochemistry, environmental science, and pharmaceuticals, are affected by the regulation. While the Beer-Lambert Law has some limitations, it remains a powerful tool for measuring the concentration of solutions. The Beer-Lambert Law is also relevant to the brewing of beer, as it can be used to determine the bitterness and other properties of the beer.
Next TopicBehavior Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










