Proton DefinitionWithin the atom's nucleus reside protons and subatomic particles. They weigh about one atomic mass unit and have a positive electric charge. Protons are responsible for an atom's mass, along with neutrons. The role of protons in influencing an element's chemical characteristics is also significant. Studying protons is crucial to physics and chemistry because it enables us to comprehend matter's composition and behavior. 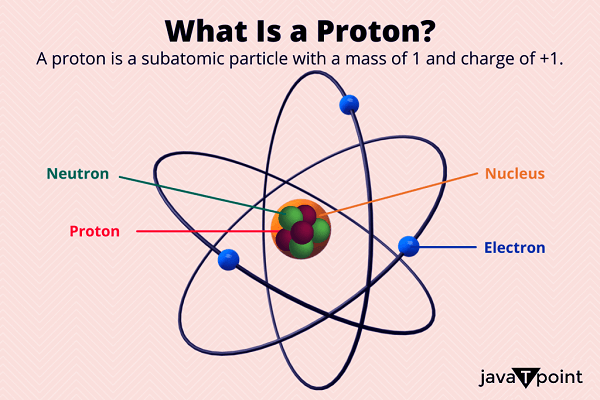
Proton CharacteristicsNumerous characteristics of protons include:
Discovery of ProtonsSeveral scientists' efforts in the late 19th and early 20th centuries led to the discovery of protons. When Eugene Goldstein noticed that cathode rays from a Crookes tube could pass through a tiny opening in the anode of the tube, he discovered a brand-new class of rays that he dubbed "canal rays." The discovery of protons was made possible by the later revelation that these rays were positive ions. The electron, which had a negative charge and was much lighter than an atom, was discovered by J.J. Thomson in 1897. This discovery made it clear that to counteract the negative charge of electrons. Atoms also needed to contain positively charged particles. 
The famous gold foil experiment, in which alpha particles were fired at a thin gold foil, was carried out in 1909 by Ernest Rutherford, Hans Geiger, and Ernest Marsden. A concentrated positive charge in the atom's center was suggested by the fact that some of the alpha particles were deflected at extreme angles. Rutherford concluded that the positive charge had to be confined in the atom's small, dense nucleus, which comprised most of its mass. Later, it was discovered that this nucleus included protons and neutrons. Rutherford hypothesized the existence of a particle in 1917 that he termed a proton and had a positive charge comparable to that of the electron. Later, it was discovered that the proton is a positively charged particle that resides in the nucleus of an atom. Structure of ProtonsProtons are subatomic particles with simple structures. They comprise two "up" quarks and one "down" quark, three different types of fundamental particles connected by the strong nuclear force. The particles we see always contain two or three quarks, fundamental particles with a fractional electric charge. The down quark has a negative charge of -1/3, while the two up quarks in a proton have a positive charge of +2/3. Combining the three quarks produces the proton's positive charge of +1. Gluons, which are particles that mediate the strong nuclear force that holds the quarks together, are also present in protons in addition to quarks. The protons and neutrons in the atomic nucleus are held together by the strong nuclear force, the strongest of the four fundamental forces. Due to the intricacy of the strong nuclear force and the challenge of researching subatomic particles at small sizes, the precise arrangement of quarks and gluons within the proton has yet to be entirely understood. However, measurements of the proton's size and its internal distribution of electric charge and magnetic moment have shed light on its structure. Atomic Proton BehaviourAtomic behavior is fundamentally influenced by protons, impacting their chemical and physical characteristics. The mass of an atom is determined by the protons and neutrons that make up its nucleus. An element's identity is determined by its atomic number and the number of protons in an atom's nucleus. For instance, all carbon atoms contain six protons, whereas all oxygen atoms contain eight. The number of protons also governs the atom's chemical characteristics. The number of electrons and the configuration of their orbits around the nucleus determine an atom's electron configuration, which controls how an atom will interact with other atoms to create chemical bonds. Protons' Significance in Physics and ChemistryThe properties and behaviour of protons are fundamental for understanding the behaviour of matter at the atomic and subatomic levels. They play a key role in physics and chemistry. The number of protons an atom has in its nucleus dictates both the chemical characteristics of the atom and its location in the periodic table. This knowledge is essential for predicting the behaviour of atom-compound compounds and comprehending the behaviour of elements. For instance, we can comprehend the behaviour of organic molecules and the biological systems they produce by understanding how the number of protons in the nucleus of a carbon atom affects its chemical behaviour and the kinds of chemical connections it may make. Protons are crucial in physics for comprehending matter's subatomic structure and behaviour. One of the core concepts in nuclear physics is the investigation of the interactions between protons, neutrons, and electrons in the atomic nucleus. Understanding radioactive decay and nuclear processes depends on how protons behave in the nucleus, affecting an atom's stability. 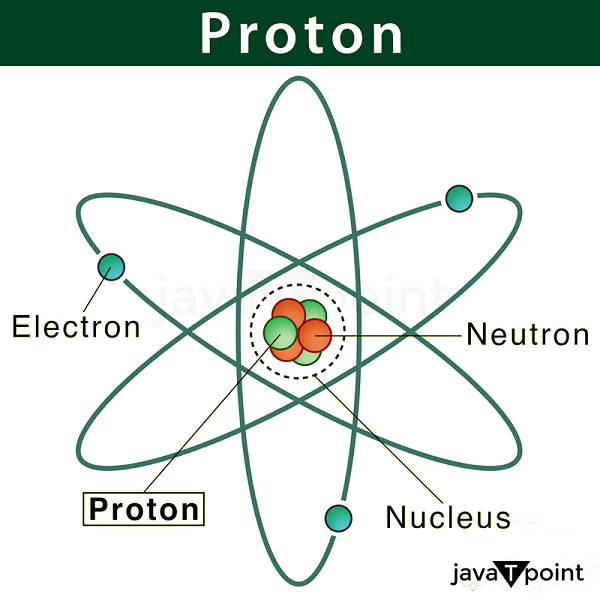
In particle physics, where they are employed to investigate the fundamental characteristics of matter and the nature of the cosmos, protons are also significant. Scientists can examine the characteristics of subatomic particles and the behavior of matter at incredibly high temperatures and densities by accelerating protons to high energies and causing them to collide with other particles. Last, knowing molecules' and materials' physical and chemical properties?crucial for a wide range of technological applications?is significant. It is crucial to develop electronic devices and technologies, such as magnetic storage and MRI, that protons behave in a way that defines the magnetic and electrical properties of materials. Proton Applications in TechnologyDue to their distinctive characteristics and behaviour, protons are used in various technological applications. The following are some of the most important uses of protons in technology: Medical ImagingMagnetic resonance imaging (MRI) uses protons, which uses powerful magnetic fields to produce images of the bodies inside organs. Radio waves can alter the body's protons, which align with the magnetic field to produce precise images of tissues and organs. Radiation therapyBy delivering radiation to the tumour while protecting neighbouring healthy tissue, high-energy protons can be utilized to treat cancer. This is referred to as proton therapy, a common therapeutic option for several types of cancer. Nuclear EnergyNuclear reactions involving protons provide energy that can be used to create electricity. Protons and neutrons are used in nuclear reactors to create heat, which is subsequently transformed into electricity through carefully controlled nuclear reactions. Particle AcceleratorsThese devices accelerate protons to extremely high energies for use in medical applications like proton treatment and for study in particle physics. Materials ScienceUnderstanding a material's qualities, such as electrical conductivity and magnetic behaviour, depends on how its protons behave. This understanding can create new materials for electronics, batteries, and other technologies. Environmental MonitoringProtons are used in the mass spectrometry method known as proton-transfer-reaction mass spectrometry (PTR-MS) to identify and quantify environmental trace gases. This method is employed to monitor atmospheric chemistry and air pollution. Overall, the special qualities of protons make them crucial for various technical uses, from energy to cancer treatment and medical imaging. 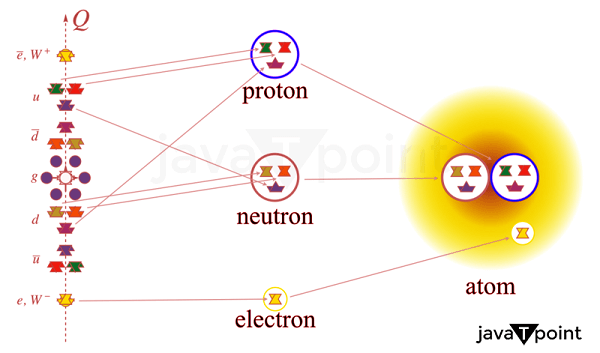
Atomic Reactions Using ProtonsNuclear processes, which include alterations to the atom's nucleus, depend critically on protons. Fusion and fission are the two primary categories of nuclear processes. In fusion reactions, a substantial quantity of energy is released as two light nuclei join to form a heavier nucleus. In infusion processes, protons combine to generate heavier elements, releasing energy through heat and light. These reactions take place in stars. A heavy nucleus is split into two or smaller nuclei in fission reactions, which release significant energy. In nuclear power plants and nuclear weapons, protons are employed in fission processes to divide heavy nuclei and liberate energy. The number of protons and neutrons in the nucleus can fluctuate during fusion and fission events, producing various elements and isotopes. This procedure is crucial for nuclear technology, energy production, and understanding how matter behaves at the atomic and subatomic levels. However, nuclear reactions can also be harmful if improperly handled due to the massive amounts of energy and radiation they might unleash. For the functioning of nuclear reactors and the handling of radioactive materials, safety precautions are consequently essential. Medical Proton TherapyHigh-energy protons are used in proton therapy, a form of radiation therapy, to cure cancer. It sends a precise radiation dose to the tumour while reducing exposure to other radiation sources. When treating tumours in delicate organs like the brain, spine, or eye, where radiation can seriously harm neighbouring good tissues, proton therapy is very beneficial. Protons can be precisely targeted, allowing for the delivery of a stronger radiation dose to the tumour while sparing neighbouring healthy tissues, lowering the possibility of adverse effects. 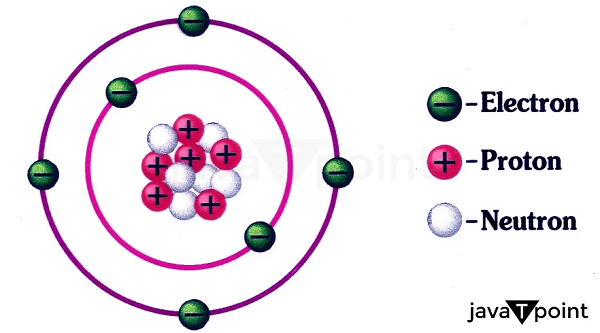
Children with cancer can benefit from proton therapy because their developing bodies are more susceptible to radiation than adults. Proton treatment can help decrease the likelihood of long-term side effects and enhance the quality of life for young cancer patients by limiting exposure to healthy tissues. Protons are often accelerated to high speeds and directed at the tumor during proton therapy treatments using a device known as a cyclotron. To ensure that the entire tumour is treated, the patient is placed on a table, and the proton beam is pointed at the tumour from various angles. Proton therapy can have negative side effects, including exhaustion, rashes on the skin, and nausea, despite being typically safe and effective. These adverse effects are often minor and disappear during treatment. Many cancer treatment facilities worldwide now provide proton therapy as a frequent treatment option for some forms of cancer. However, due to its recent development and specialization, all cancer treatment facilities may not provide proton therapy or cover all insurance plans. Acidity and Proton Exchange in ChemistryFundamental chemistry principles that deal with the transfer of protons between molecules include proton exchange and acidity. Proton exchange is often used to transfer protons across molecules in aqueous solutions. Acidity is determined using the pH scale and relates to a substance's capacity to contribute protons. The pH scale ranges from 0 to 14, with a neutral pH of 7, an acidic pH of less than 7, and a basic pH of more than 7. Bases are chemicals with a high pH and can take protons, while acids are substances with a low pH and can donate protons. Many chemical reactions, such as acid-base and redox, depend on proton exchange reactions. Protons are transferred from an acid to a base during an acid-base reaction, creating a fresh acid and fresh base. Protons frequently participate in the exchange of electrons between molecules in redox processes. The ease with which an acid or base can give or take protons is correlated with its strength. While weak acids, like acetic acid (CH3COOH), donate protons less quickly and have a higher pH, strong acids, like hydrochloric acid (HCl), readily donate protons and have a low ph. 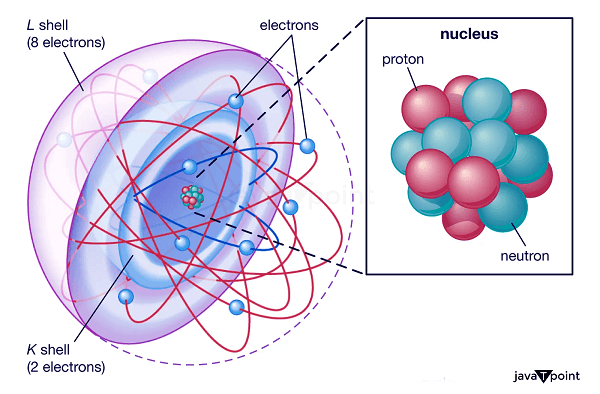
Proton exchange reactions are critical for enzyme-catalyzed reactions and the control of biological activities, and they are also significant in biochemistry. To maintain ideal conditions for biochemical reactions, the pH of biological fluids such as blood and cytoplasm is strictly controlled. Therefore, knowing proton exchange and acidity is crucial for developing new medications and materials and understanding chemical and biological processes. Physics of proton conduction in materialsThe transport of protons through a material is known as proton conduction, and it is essential for many technological applications, such as fuel cells, batteries, and sensors. Proton conduction in solid-state materials can happen through several mechanisms, such as the Grotthuss, vehicular, and hopping mechanisms. In contrast to the vehicular process involving protons being transported by water molecules or other mobile species, the Grotthuss mechanism includes the rapid transfer of protons through a network of hydrogen bonds. The protons diffuse between locations in the material as part of the hopping action. Fuel cells, which transform chemical energy into electrical energy, depend heavily on proton conduction. At the anode of a fuel cell, hydrogen gas is oxidized, liberating protons and electrons. The protons then travel to the cathode via a proton-conducting membrane, combining with oxygen to generate water. An electrical current is created as the electrons go via an external circuit. Batteries also use proton-conducting materials, which are essential for the movement of ions between the electrodes. Compared to conventional lithium-ion batteries, proton-conducting electrolytes may have a higher power density, quicker charging times, and improved safety. Proton-conducting materials are also utilized in sensors and actuators, fuel cells and batteries, where they can be used to monitor changes in temperature, humidity, or other environmental factors. The goal of proton conduction research is to create novel materials that are more proton conductive, stable, and effective. The advancement of innovative technologies that can lessen our reliance on fossil fuels and increase the effectiveness and sustainability of our energy systems depends on the results of this research.
Next TopicProtoplasm Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










