Pharmacopoeia DefinitionDefinitionA pharmacopoeia is a detailed manual that provides information regarding medicinal pharmaceuticals' preparation, usage, and qualities. The concept's origins may be traced back to ancient Greece when it was used to denote a collection of medicinal recipes and formulas used by physicians and chemists to cure various diseases and disorders. 
Pharmacopoeias gained popularity and standardization throughout time, representing the increasing complexity of medical treatments and the need for greater precision and consistency in medicine manufacturing and distribution. Pharmacopoeias are now mainly used by healthcare professionals worldwide to ensure that pharmaceuticals are safe, effective, and high-quality. One of the most important characteristics of pharmacopoeias is their focus on standardization. It includes developing accurate and thorough medication production and testing methods and establishing quality control mechanisms to assure consistency in drug composition, purity, and effectiveness. Standardization is essential for assuring medication safety and efficacy since tiny differences in drug composition or quality can significantly influence patient outcomes. Pharmacopoeias are essential in drug control and supervision. In addition to standardization, Pharmacopoeias are used by governments and regulatory agencies worldwide to ensure medication safety and effectiveness and to approve novel pharmaceuticals for use in clinical settings. It guarantees that pharmaceuticals are extensively tested and reviewed before they are made available to patients, and it aids in preventing the use of dangerous or inefficient therapies. HistoryThe science of medicines and their creation, pharmacopoeia, has a long and fascinating history. This field's beginnings may be traced back to ancient times when physicians and healers developed medicines and treatments based on natural elements. These early efforts prepared the way for the development of the first pharmacopoeias, which collected data on the components, production techniques, and therapeutic applications of numerous medications. The Chinese "Shennong Bencao Jing," written around 2000 BCE, is the first known pharmacopoeia. This literary work described hundreds of medicinal plants and their applications and laid the groundwork for creating traditional Chinese medicine. Similar books were produced in various world regions, such as the Greek "Materia Medica" and the Arabic "Kitab al-Saydalah," compiled in the first millennium CE. 

Pharmacopoeias were more standardized and structured during the Middle Ages, reflecting the increased complexity of medical practice. The Persian physician Rhazes wrote the "Liber pantegni" (Book of General Medicine) in the 9th century, one famous example. The book was a complete reference to medical knowledge, explaining disorders, treatments, and the pharmacological properties. Pharmacopoeias became increasingly important for ensuring the safety and quality of medicines as transportation and trade developed throughout the Renaissance era. The College of Physicians in London produced the first formal pharmacopoeia in Europe in 1546. The "Pharmacopoeia Londinensis" publication stated accepted drugs and their manufacturing processes and was updated regularly to reflect discoveries and enhancements. Pharmacopoeias continued to evolve in the 17th and 18th centuries, with new works issued in different countries. The "Pharmacopoeia Amstelredamensis," compiled by the Amsterdam College of Physicians in 1636, was among the most significant. This book was famous for emphasizing exact dosage and using Latin as the official medical terminology. Researchers began looking at medications' chemical composition and effects in the 19th century, and pharmacology was born as a separate subject. It encouraged the creation of new pharmacopoeias that represented these developments and resulted in more precise drug analysis and estimation procedures. One of the most significant was the "United States Pharmacopoeia" (USP), first released in 1820 and now an important source of information about drugs and standards. Pharmacopoeias still play a significant role in medicine today, establishing guidelines for the safe and efficient administration of medicines. National or international organizations produce the most modern pharmacopoeias and provide complete information regarding purity, quality control, and drug formulation. They are also an asset for academics and medical experts, who utilize them to discover important details like possible drug combinations and side effects. In conclusion, the pharmacopoeia's rich and complex history brings together a piece of art that combines many centuries and civilizations. The demands and goals of doctors, scientists, and patients together have affected the evolution of this field, from early herbal medicines to modern drugs. Pharmacopoeias will surely be important as medicine develops in ensuring that medicines are dependable, effective, and safe for future generations. Contents of PharmacopoeiaThe contents of a pharmacopoeia may vary slightly depending on the country or region, but they generally cover the following topics.
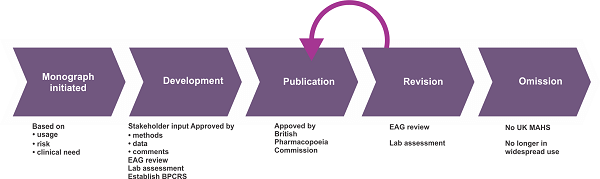
A pharmacopoeia's contents offer healthcare professionals a thorough reference for medicines and their uses in treatment. The formulary, dosage forms, packaging, labeling rules, methods of analysis, and reference standards give precise information on the quality and purity of medications. In contrast, the monographs, methods of analysis, and reference standards guide their appropriate use. Pharmacopoeias are a crucial component in the growth and management of the healthcare sector and are a key instrument for guaranteeing the effectiveness and safety of medicines. Importance of PharmacopoeiaPharmacopoeia's significance is mostly due to its function maintaining the uniformity and quality of pharmaceutical goods. Drugs must adhere to strict requirements outlined in the pharmacopoeia before being sold or used in clinical settings. These requirements, such as medication effectiveness, identification, and durability, cover numerous characteristics. By following these standards, pharmaceutical companies can ensure that their goods are reliable, consistent, and safe. Another crucial role of pharmacopoeia is to protect public health by ensuring that drugs are safe. Pharmacopoeias provide guidelines for the identification, purity, and quality of active pharmaceutical ingredients (APIs) used in drug formulations. These guidelines help to prevent the use of substandard or adulterated drugs that could harm patients. Pharmacopoeia also plays a vital role in drug regulation. Regulatory agencies, such as the FDA in the US, rely on pharmacopoeia standards to assess drug quality and approve new drugs. By using pharmacopoeia standards, regulatory agencies can ensure that drugs are safe and effective before they are approved. Pharmacopoeias also ensure the right preparation and administration of medicines. In addition to dosage forms like pills, capsules, and injections, the pharmacopoeia also contains instructions for preparing and testing pharmaceuticals. These directions help ensure the proper preparation of medications, lowering the possibility of mistakes and guaranteeing that patients receive the appropriate medication dosage. Pharmacopoeias are also important in the creation of new drugs. Pharmaceutical companies use pharmacopoeia standards to create and test new drugs. These drug quality, purity, and potency guidelines help ensure their safety and effectiveness before authorization. In conclusion, pharmacopoeia's significance cannot be emphasized, and it will continue to be a crucial instrument in the pharmaceutical industry for years to come. Drawbacks of PharmacopoeiaThe vast amount of medications that need to be evaluated and controlled is one of the main problems against pharmacopoeia. Every year, more and more new drugs are released into the market, and each one needs to undergo quality, purity, and safety checks. It significantly burdens regulatory organizations and may cause delays in the licensing procedure. Globalization of the pharmaceutical industry is yet another important obstacle. With many drugs now being manufactured in developing countries, there is a growing concern about the quality and safety of these drugs. A challenging endeavor requiring tremendous resources and knowledge is ensuring that medicines worldwide adhere to the same criteria. In addition, the speed of technological advancement brings about new difficulties for pharmacopoeia. For example, creating new drug delivery systems like nanoparticles and microspheres brings thorough testing and regulatory difficulties. Developing new testing procedures and standards for these technologies can be time-consuming and costly. The growing complexity of medicines themselves is another problem. Drugs may need specialized testing and regulation as they become increasingly specific and personalized. Pharmacopoeia may find it increasingly challenging to keep up with new medications and technological advancements, perhaps putting patients in danger. Pharmacopoeia's reliance on animal experimentation is one of its biggest flaws. Many people think that using animals for research is needless and inhumane. In contrast, others argue it is essential in the quest for safe and effective medications. In addition, it can be costly and time-consuming to utilize animals for drug testing. Given that animals don't always respond to medications in the same manner that people do, it could occasionally be unreliable. As a result, there is a growing interest in creating alternative testing techniques, such as computer simulations and in vitro testing. The problem of the cost comes last. Testing and regulating pharmaceuticals may be expensive, and this expense is ultimately passed on to consumers. The burden on patients, especially those with chronic diseases that require continued treatment, can be severe due to increasing drug prices.
Next TopicPlant Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










