Unit Cell DefinitionUnit cells are the building blocks of crystals, which are found in various materials like metals, minerals, and polymers. A unit cell is a basic structural unit of a crystal that is repeated in three dimensions to form the entire crystal lattice. The properties of a crystal depend on the structure of its unit cell, and the unit cell's characteristics define the crystal's behavior. 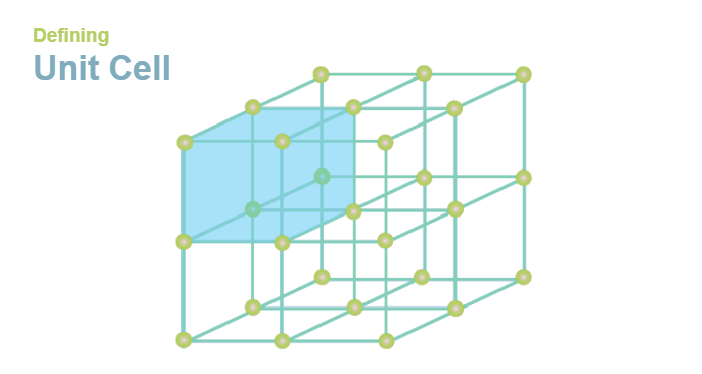
Characteristics of a Unit CellSymmetryThe unit cell must have a symmetry that is consistent with the symmetry of the crystal. The symmetry of the unit cell is determined by the crystal system and the space group. There are seven different crystal systems, and they all have different symmetry components. These symmetry elements typically include rotational axes, mirror planes, and inversion centers. The space group describes the specific arrangement of atoms within the unit cell and their symmetry operations. Lattice ParametersThe unit cell's size and form are determined by the lattice parameters. The three lattice parameters are the lengths of the three edges of the unit cell (a, b, and c) and the angles between them (?, ?, and ?). The lattice parameters are also determined by the crystal system and the space group. They are measured in units of length, such as angstroms or nanometers. Atomic PositionsThe atomic positions describe the location of atoms within the unit cell. The atomic positions are also specified by the crystal system and the space group. They are typically given in fractional coordinates, which represent the position of the atom relative to the lattice parameters. The fractional coordinates are usually expressed as (x, y, z), where x, y, and z are the fractional coordinates along the three lattice parameters. DensityThe density of the unit cell is the mass per unit volume of the crystal lattice. The density is determined by the atomic weight of the atoms in the unit cell and the volume of the unit cell. The density of a crystal is an important physical property that affects its mechanical, optical, and electronic properties. Packing EfficiencyThe amount of space taken by the unit cell, which is occupied by atoms in terms of packing efficiency, is expressed as a percentage. The packing efficiency is determined by the sorting of atoms within the unit cell. The highest packing efficiency is achieved by the closest packing of atoms. The maximum packing efficiency is 68% for cubic closest packing and 74% for hexagonal closest packing. The packing of atoms within a unit cell determines the crystal's density and stability. The most common packing arrangements are cubic, tetrahedral, octahedral, and hexagonal close packing. Crystal HabitThe crystal habit determines the characteristic shape of a crystal. The crystal habit is measured by the growth of the crystal during crystallization. The crystal framework, the space category, and the lattice parameters all have a huge impact on crystal habit. The crystal habit is an important physical property that can be used to identify minerals and other crystalline substances. The crystal system is the classification of crystals based on their symmetry and the shape of their unit cell. CleavageThe propensity of a crystal to fracture along particular weak points is known as cleavage. The cleavage of a crystal is determined by the arrangement of atoms within the crystal lattice. The cleavage planes are typically parallel to planes of high atomic density. Cleavage is an important physical property that can be used to identify minerals and other crystalline substances. TwinningTwinning is the occurrence of two or more crystals that share a common lattice but have different orientations. Twinning occurs when crystals grow under conditions that cause them to adopt different orientations. Twinning is an important physical property that can be used to identify minerals and other crystalline substances. Stacking FaultsStacking faults are defects that occur when the layers of atoms in a crystal lattice are not perfectly aligned. Stacking faults occur when atoms are displaced from their ideal positions within the lattice. Stacking faults can affect the mechanical, optical, and electronic properties of a crystal. DislocationsDislocations are defects that occur when there is a deviation from the ideal periodicity of the crystal lattice. Dislocations can occur due to defects in the crystal structure or external stresses. Dislocations can affect the mechanical and electronic properties of a crystal, and they can also affect the growth and deformation of crystals. ShapeThe shape of the unit cell determines or defines the overall shape the crystal is going to take. The most common unit cell shapes are cubic, tetragonal, orthorhombic, hexagonal, rhombohedral, and monoclinic, among many others. The shape of the unit cell is defined by the lengths of its edges and the angles between them. Edge LengthThe edge length of the unit cell is the distance between two adjacent unit cells in the crystal lattice. The edge length is crucial for determining the overall density of the crystal, as the number of existing atoms in a unit cell is proportional to the total volume of the unit cell. Coordination NumberThe quantity of atoms around the core atom in a unit cell is known as the coordination number. The crystal's hardness, melting temperature, boiling point, and a variety of other chemical and physical properties are significantly influenced by the coordination number. Miller IndicesMiller indices are used to identify the orientation of planes within a crystal. The Miller indices are used to describe the position of atoms within the unit cell and their arrangement in the crystal lattice. VolumeThe volume of the unit cell is the total space occupied by the atoms within the cell. The unit cell's volume is essential in determining the density of the crystal and the number of atoms within the crystal lattice. Types of a Unit CellThe concept of unit cells is an important topic in the study of crystallography, which is the science of studying the properties and structure of crystals. A unit cell is the smallest repeating unit of a crystal lattice, and it is used to describe the structure of a crystal. Unit cells can be of different types; however, the three main types include simple-cubic, body-centered, and face-centered unit cells. 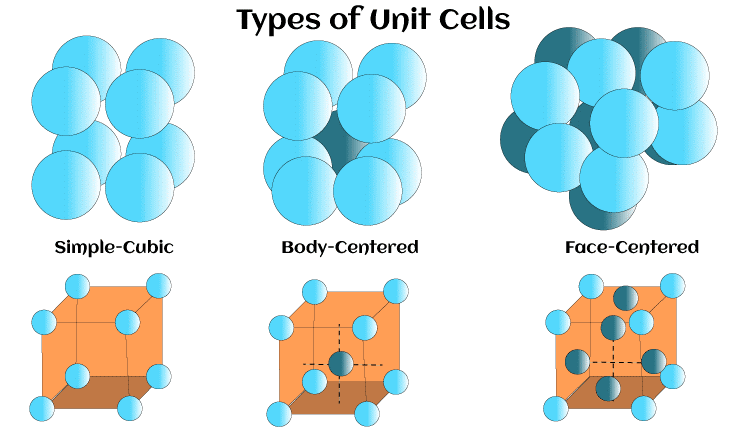
Simple Cubic Unit CellThe simplest form of the unit cell is the simple cubic one, which is a cube with atoms present at every angle. Each atom is connected to the next set of atoms, which creates a simple cubic lattice. The synergizing number of each atom is 6, which means that each atom has 6 nearest neighbors. The simple cubic unit cell is the least dense of all the unit cells, and it is not commonly found in nature. Some examples of materials that exhibit a simple cubic structure include:
Body-Centered Cubic Unit CellThe body-centered cubic (BCC) unit cell consists of a cube with atoms at each corner and an additional atom at the center of the cube. This type of unit cell is denser than the simple cubic unit cell, with a coordination number of 8. The BCC unit cell is found in a number of metals, including iron and tungsten, among many others. Some examples of materials that exhibit a body-centered cubic structure include:
Face-Centered Cubic Unit CellThe face-centered cubic (FCC) unit cell is similar to the BCC unit cell, but it has an additional atom at the center of each face of the cube. This type of unit cell is the most common one in nature, and it is found in a number of metals, including copper, silver, etc. The coordination number of each atom in the FCC unit cell is 12, which means that each atom has 12 nearest neighbors. Some examples of materials that exhibit a face-centered cubic structure include:
Hexagonal Close-Packed Unit CellThe hexagonal close-packed (HCP) unit cell consists of a hexagonal prism with atoms at each corner and an additional atom at the center of each hexagonal face. This kind of unit cell can be located in a number of metals, including zinc, titanium, etc. Its coordination number is 12. Some examples of materials that exhibit a hexagonal close-packed structure include:
Diamond Cubic Unit CellThe diamond cubic unit cell is a variation of the face-centered cubic unit cell, in which the atoms are arranged in a diamond-like pattern. This kind of unit cell is found in diamond, silicon, and germanium. Its coordination number is 4. Some examples of materials that exhibit a diamond-cubic structure include:
Sodium Chloride Unit CellThe sodium chloride unit cell consists of a cube with sodium ions at each corner and chloride ions at the center of each face. This type of unit cell is found in sodium chloride (table salt) and a number of other ionic compounds. Its coordination number is 6. Some examples of materials that exhibit a sodium chloride structure include:
Fluorite Unit CellThe fluorite unit cell consists of a cube with calcium ions at each corner and fluoride ions at the center of each face. This type of unit cell is found in fluorite (typically calcium fluoride) and a number of other ionic compounds. Its coordination number is 8. Some examples of materials that exhibit a fluorite structure include:
Application of Unit Cell in Various FieldsThe study of crystals has played a vital role in the advancement of modern science and technology. One of the fundamental concepts in the study of crystals is the unit cell. A unit cell is the smallest repeating unit of a crystal lattice that contains all the information about the crystal structure. The unit cell concept is used in many fields, including chemistry, materials science, geology, and solid-state physics. Some notable unit cell applications can be found in the following areas: CrystallographyThe most obvious application of the unit cell concept is in crystallography. The unit cell concept allows crystallographers to describe the crystal structure of a material in a concise and accurate way. For example, the diamond crystal lattice consists of repeating units of carbon atoms arranged in a face-centered cubic (FCC) structure. Eight atoms make up the unit cell of a diamond, and each one is positioned in the middle of each face and at one of the cube's four corners. Material ScienceUnit cells are also important in material science. Material scientists use the concept of the unit cell to design and engineer new materials with desired properties. For example, the unit cell concept is used to understand the properties of metals, ceramics, and polymers. The crystal structure of metals determines their mechanical, electrical, and thermal properties. By altering the unit cell parameters, material scientists can modify the crystal structure and create new materials with specific properties. For example, the unit cell of austenitic stainless steel consists of a face-centered cubic (FCC) structure of iron atoms with carbon, nickel, and chromium atoms occupying interstitial sites. GeologyGeologists also use the unit cell concept to study the crystal structures of minerals. Minerals are naturally occurring solids with a defined chemical composition and crystal structure. The unit cell of a mineral provides information about its crystal symmetry, crystal habit, and physical properties. Solid-State PhysicsThe concept of the unit cell is also used in solid-state physics to understand the behavior of electrons in crystals. The electronic structure of a crystal is determined by the arrangement of atoms in the unit cell. By understanding the electronic structure, physicists can predict the properties of materials, such as conductivity, magnetism, and optical properties. Molecular BiologyUnit cells are also used in molecular biology to study the crystal structures of biological macromolecules such as proteins and nucleic acids. The unit cell of a protein crystal provides information about its three-dimensional structure, which is important in drug discovery and design. NanotechnologyThe concept of the unit cell is also important in nanotechnology. The unit cell provides a fundamental building block for the design and synthesis of nanomaterials with specific properties. Solar EnergyThe concept of the unit cell is also used in the design of solar cells. The unit cell of a solar cell consists of a semiconductor material such as silicon, which absorbs photons and generates electrons. By optimizing the unit cells parameters such as bandgap and doping level, solar cell designers can maximize the efficiency of the solar cell. ConclusionIn conclusion, the concept of the unit cell is fundamental in the study of crystals and their properties. Unit cells are used in many fields, including crystallography, material science, geology, solid-state physics, molecular biology, nanotechnology, solar energy, and medical imaging. By understanding the unit cell parameters, scientists and engineers can design and synthesize new materials with tailored properties for specific applications. The unit cell concept is essential for advancing modern science and technology and has many real-life applications or uses.
Next TopicVertically Opposite Angles Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










