Solubility DefinitionSolubility is a property of a material that describes its ability to dissolve in a solvent. It is an important physical property often used to characterize a substance and its behaviour in an aqueous solution. The maximum quantity of solute that may dissolve in a given amount of solvent at a given temperature and pressure is known as solubility. The solubility of a substance is determined by its chemical and physical properties, such as molecular structure, molecular size, polarity, and temperature. 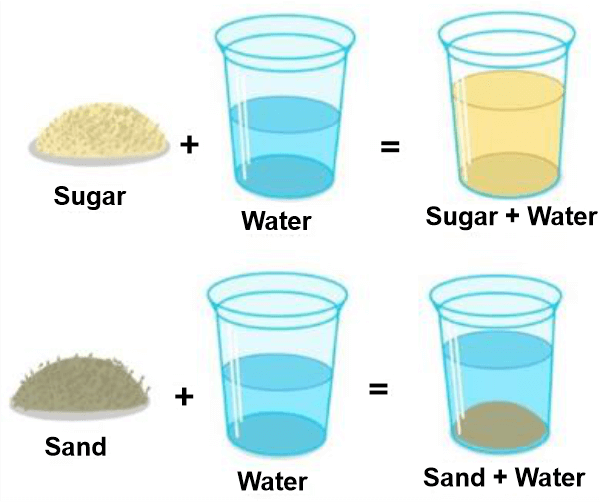
Types of SolubilitySolubility can be divided into four types: Molecular, Ionic, Metallic, and Non-Ionic. This is known as molecular solubility when the solute's molecules dissolve in the solvent. Ions of the solute are dissolved in the solvent when there is ionic solubility. Metallic solubility occurs when metal ions of the solute dissolve in the solvent. Non-ionic solubility occurs when the solute does not dissolve in the solvent but instead forms a colloidal suspension. 1. Molecular Solubility The most prevalent type of solubility, known as molecular solubility, is normally measured in moles of solute per litre of solution (M). The solubility of molecular substances is influenced by molecular size, polarity, and temperature. The solubility of molecular substances increases with increasing temperature and decreases with increasing molecular size. Nonpolar molecules are more soluble in nonpolar solvents than polar molecules, and vice versa for polar molecules in polar solvents. 2. Ionic Solubility Ionic solubility occurs when ions of the solute dissolve in the solvent. This solubility is very important in the pharmaceutical and medical fields, as many drugs and medicines are ionic compounds. The type of ions in the fluid affects how easily ionic substances dissolve. Ionic compounds that contain ions of the same charge are generally more soluble than ionic compounds that contain ions of opposite charges. The solubility of ionic compounds is also influenced by the polarity of the solution, as ions of the same charge are more soluble in polar solvents than in nonpolar solvents. 3. Metallic Solubility Metallic solubility occurs when metal ions of the solute dissolve in the solvent. This type of solubility is mainly used in the production of metals, such as copper and iron, as well as in the manufacturing of alloys. The solubility of metals is determined by the nature of the metal, its oxidation state, and the nature of the solvent. The solubility of metals increases with increasing temperature and decreases with increasing oxidation state. 4. Non-Ionic Solubility Non-ionic solubility occurs when the solute does not dissolve in the solvent but instead forms a colloidal suspension. The solubility of non-ionic substances is determined by their molecular size and polarity, as well as the nature of the solvent. Non-ionic substances are more soluble in nonpolar solvents than in polar solvents. Non-ionic substances are also more soluble in solvents with a higher dielectric constant than solvents with a lower dielectric constant. 
Factors Affecting Solubility
Solubility of Liquids in LiquidsThe solubility of liquids in liquids is the ability of one liquid to dissolve in another. This process is known as Miscibility, which is important for various industrial and biochemical processes. When one liquid is soluble in another, the two liquids form a homogenous mixture, which means the two liquids mix evenly and uniformly. This is because the molecules of the two liquids are attracted to each other, so they move freely throughout the mixture. The ability of one liquid to dissolve in another is called Solubility. The solubility of a liquid in another liquid is determined by the relative strengths of the intermolecular forces between the two liquids. The solubility increases with increasing power. For instance, water is a polar molecule, which means that the positive and negative charges are spread unevenly throughout the molecule. This makes it very soluble in other polar molecules, like alcohol. On the other hand, oil is a nonpolar molecule, meaning the positive and negative charges in the molecule are evenly distributed. This makes it less soluble in polar molecules like water. The temperature also affects solubility. As the temperature rises, a liquid will often become more soluble in another liquid. This is because as the molecules' kinetic energy rises, their range of motion expands. They can combine more easily as a result. In general, the solubility of a liquid in another liquid is a function of the relative strengths of the intermolecular forces between the two liquids. When two liquids have strong, attractive forces, they will be more soluble in each other. When the attractive forces are weak, the two liquids may not mix well or may not mix at all. It is also important to consider the temperature when determining solubility, as higher temperatures generally increase solubility. Understanding the solubility of liquids in liquids can help scientists and engineers design effective products and processes. Solubility of Solids in LiquidsThe solubility of solids in liquids is a key phenomenon in many chemical, biological, and industrial processes. A solid material can dissolve in a liquid, forming a homogeneous solution. The intermolecular interactions between the two materials and the temperature, pressure, and other external conditions affect a solid soluble in a liquid. Solubility is an important property to consider when designing and manufacturing products, as it can impact the rate at which a product can dissolve and the concentration of the solution that is formed. Many solids are soluble in water, which can be seen in everyday life, such as when sugar is added to a cup of tea. Other solids such as alcohols, ketones, esters, and hydrocarbons are also soluble in organic solvents. The presence of other substances in the solution might also affect a substance's solubility. For example, the presence of an electrolyte such as salt can increase the solubility of a solid in a liquid. The type of ions and the electrolyte concentration can affect the material's solubility. Likewise, the presence of a surfactant, or wetting agent, can also increase the solubility of a solid in a liquid. Temperature can considerably impact how soluble a solid is in a liquid. The solubility of solids often rises with temperature in general. This is why it is important to consider the temperature of the solution when designing a product, as it can impact the rate at which the solid material dissolves. Pressure can also affect the solubility of a solid in a liquid. Generally, increasing the pressure of a solution increases its solubility, while decreasing the pressure decreases its solubility. This can be seen in the fact that many materials are more soluble in liquid carbon dioxide at higher pressures, such as at the bottom of the ocean or in pressurized containers. When creating items, it's crucial to consider their solubility in liquids. The presence of additional substances in the solution and the solution's temperature and pressure might affect how soluble a solid is. Understanding the solubility of solids in liquids makes it possible to design more efficient products and processes. Solubility of Gases in LiquidsThe capacity of gas to dissolve in a liquid and create a homogenous solution is known as the solubility of gases in liquids. Numerous biological and commercial activities and daily living depend on this process. Various physical and chemical processes, such as molecular size, polarity, intermolecular forces, and temperature, determine a gas's solubility in a liquid. The solubility of a gas in a liquid will depend on the gas's pressure, the liquid's temperature, and the liquid's chemical composition. Molecular size is an important factor in determining the solubility of a gas in a liquid. Gases with smaller molecules are more soluble in liquids than gases with larger molecules. Smaller molecules can more easily pass through the liquid's surface and mix with the liquid. Molecular polarity is also important in determining a gas's solubility. Polar molecules are more soluble in polar liquids than nonpolar molecules due to the attraction between the polar molecules and the liquid. Nonpolar molecules are more soluble in nonpolar liquids. 
Intermolecular forces are also important in determining gas solubility. These forces are the attractive and repulsive forces between molecules. Attractive forces help to increase solubility, while repulsive forces decrease solubility. Temperature is another important factor in determining the solubility of a gas in a liquid; generally, as the temperature increases, the solubility of a gas in a liquid increases. The molecules move faster and mix more easily with the liquid. The solubility of a gas in a liquid can also be affected by the presence of other substances. For example, the presence of an acid or a base can increase or decrease the solubility of a gas in a liquid. The solubility of a gas in a liquid is an important factor in many industrial and biological processes and everyday life. Various physical and chemical processes, such as molecular size, polarity, intermolecular forces, and temperature, determine a gas's solubility in a liquid. The solubility of a gas in a liquid can also be affected by the presence of other substances. Understanding the solubility of a gas in a liquid is essential for a successful industrial or biological process. Some Other Important Terms
Why is Solubility Important?Solubility is important because it determines the amount of a substance that can be dissolved in a solvent. Solubility is also important for many practical applications, such as pharmaceuticals, food, and cosmetics. For example, solubility can be used to determine drug delivery times, flavour release, and stability of ingredients. Additionally, solubility can be used to determine the best methods for separating and purifying compounds. 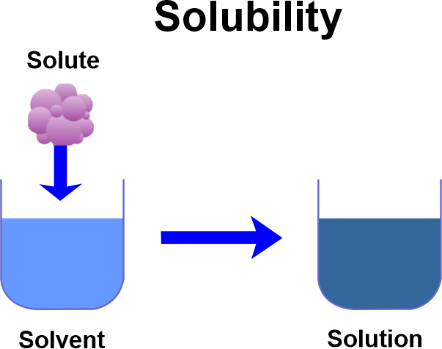
How is Solubility Helpful for Pharmaceuticals?Solubility is important for pharmaceuticals because it can be used to determine the rate at which the body absorbs a drug. Solubility also affects the stability of the drug in the body, which is important for safety and efficacy. Additionally, solubility can be used to determine the best methods for separating and purifying compounds.
Next TopicSuspension Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share









