Vapour Pressure DefinitionThe pressure that results from a liquid (or solid) condensing on its surface is referred to as vapour pressure. This pressure is made in a confined receptacle at a specific temperature during a thermodynamic equilibrium condition. The vapour pressure shows how quickly a liquid is vaporizing. The liquid meets its boiling point when the vapour and gas pressure are equal. In this writing, we will discover the characteristics of liquid vapour pressure. 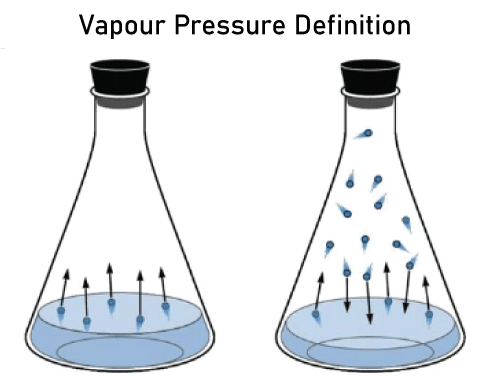
Vapour PressureThe propensity of a substance to transform into a vapour condition is known as vapour pressure. Also, the pressure that a liquid's vapour exerts on its condensed stages in a confined system is known as vapour pressure. Any liquid's air pressure rises in direct proportion to its temperature. Several methods can determine a liquid's vapour pressure. Placing a manometer inside a flask or other confined receptacle is the easiest way to measure vapour pressure. In all substances, there is a tendency towards evaporation. At the liquid's surface, the vaporization process takes place. In the liquid state, molecules from the liquid's surface can flee into space if their kinetic energy exceeds the pull force between molecules. The action is referred to as "evaporation." If evaporation occurs in a confined container system, liquid vapours remain in touch with the liquid's surface. The condensing of molecules also carries out a constant random motion, like gas molecules. As a result of the collisions between the molecules during these motions, including those with the container walls, they lose energy and transform back into liquids. The procedure is referred to as condensation. Condensation and EvaporationCondensation and evaporation are ongoing processes. Consequently, evaporation and condensation reach a state of balance at a fixed temperature after some time. The condition is steady at a fixed temperature when the quantity of molecules in the vapour comes to equilibrium. The intermolecular force of pull increases with temperature, which affects the liquid's vapour pressure. When the temperature rises, the vapour pressure of a liquid grows and diminishes. However, as time goes on, the condensation rate and molecules present in the liquid phase increase. When that happens, the rate of condensation and vaporization are identical. The pressure exerted by molecules in this situation, as shown by the manometer, is called the liquid's vapour pressure. The pressure the liquid's surrounding vapours exerts is known as vapour pressure. Vapour Pressure CharacteristicsVapour pressure is solely influenced by temperature. No matter how much liquid is in the container, one liter or 40 liters of liquid will have the same vapour pressure at the same temperature. Temperature and vapour pressure have an exponential relationship, which indicates that as temperature increases, so does vapour pressure. A pure liquid experiences more vapour pressure than a liquid in solution. Vapour Pressure Influencing FactorsVapour pressure relies on several variables which are as follows: 1. Liquid's NatureThe liquid's intermolecular forces are used to describe its structure. The gaseous pressure will decrease as the strength of the intermolecular interactions increases, in other words. 2. Solute ConcentrationA substance in the liquid will greatly lower the vapour pressure. The decline in vapour pressure also varies depending on the solute content. 3. Temperature EffectThe liquid's kinetic energy increases as its temperature rises, and because of this increase in rotational energy, molecules are more likely to escape, which increases vapour pressure. As a result, we can determine that temperature and air pressure are proportionate. 4. Humidity has No Effect on Vapour PressureTemperature is the only factor influencing the vapour pressure for a specific quantity of air-water vapour. Only when every other element is consistent will humidity have an effect. Therefore, avoid mixing up the impact of temperatures and humidity. 5. VolumeThe capacity of the receptacle typically has little impact on vapour pressure. As we already know, the area's liquid and vapour will be in balance. Consider that the volume of the surface is divided into infinite component volumes, changing its volume and declaring it decreased. As a result, some of the container's vapour will also transform into a liquid form, and some of the liquid will inevitably turn into its vapour form as the amount increases. 6. Area of SurfaceTypically, surface size has no impact on vapour pressure. Factors affecting Liquid Vapour PressureHowever, the following elements impact a liquid's stable vapour pressure: 1. Forces of Intermolecular AttractionIntermolecular forces are any forces, such as powers of attraction or repulsion, that facilitate a relationship between atoms. For instance, the covalent connection, which involves exchanging electron pairs between atoms, is much more powerful than the intermolecular force between nearby molecules. 2. The Liquid's Present VolumeThe amount of the liquid does not influence the equilibrium vapour pressure of the liquid. While maintaining a steady temperature, a liquid's volume can be shifted, but its equilibrium vapour pressure won't move. 3. The Liquid's TemperatureWhen a fluid is in equilibrium, its vapour pressure rises as its temperature rises or the intermolecular forces of attraction diminish. FAQs on the Vapour Pressure1. What are liquid's properties?Answer: Liquid's properties are:
2. Difference between vapour pressure and boiling pointAnswer:
3. What is the vapour pressure unit?Answer: The most utilized unit for it is torr. 4. Which has the highest vapour pressure?Answer: The substance with a low boiling point will have a highvapour pressure at normal temperature.
Next TopicAction Potential Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










