Lattice Energy DefinitionIntroductionElectropositive metals and electronegative nonmetals interact to generate solid ionic compounds. Lattice energy, a sort of potential energy measured in kJ/mol, is a notion that is involved in both the formation & dissolution of such compounds. Ionic compounds' fixed locations of anions and cations are maintained by lattice energy. Depending on our viewpoint, there are two ways we might explore this concept further. The crystalline structure of ionic substances holds the key to comprehending this idea. Each charged ion interacts with its oppositely charged counterparts thanks to its robust, rigid constitution. These intense interactions account for the high melting & boiling temperatures that distinguish ionic compounds. One mole of a solid ionized substance can be formed when gaseous ions react, releasing a certain amount of energy. Lattice energy can also be used to describe the energy required to facilitate the dissociation of one mole of the solid ionic compound into its component gaseous ions. The lattice energy of a particular ionic molecule might either be a positive or negative number, depending on the definition we choose. 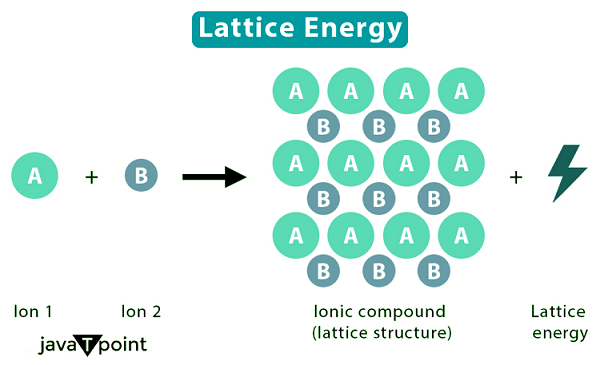
Factors Affecting Lattice Energy1. The charges associated with the constituent ions carried, as indicated by the variables Q1 as well as Q2. The lattice energy rises when the ionic charge is increased. Accordingly, ions with higher charge intensities will result in ionic compounds with higher lattice energies. In consequence, ions with lower charges cause their compounds' lattice energies to fall. 2. The separation of the ions' component parts (expressed by the variable R) Lattice energy diminishes when the distance variable is increased. In essence, the greater distance between bigger ions results in ionic compounds with decreased lattice energies. Larger lattice energies are produced in the ionic compounds of smaller ions. 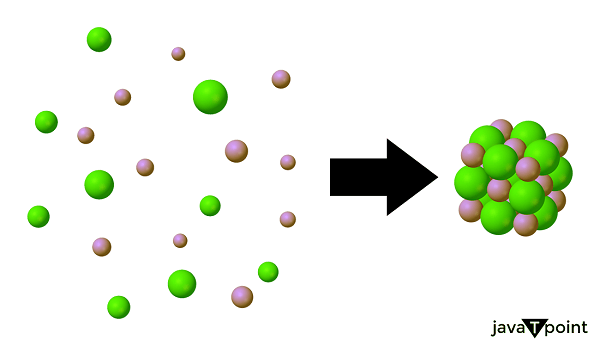
The Uses of Lattice EnergyBorn-Haber CycleChemists may analyze reaction energies using the Born-Haber Cycle, which involves the Born-Haber Cycle lattice energy. This cycle is frequently used as a reference when looking into the synthesis of ionic compounds utilizing various elements. By dissecting the total response process into many parts, it becomes more understandable. Hess's Law, which asserts that it is possible to identify general energy changes by first examining individual stages and then integrating their effects, is the basis for this kind of chemical reaction analysis. We can solve for lattice energy when the other variables are entered since it is a component of the Born-Haber Cycle equation. The formula is as follows: The heat of formation is equal to the sum of the ionization energies, dissociation energies, lattice energies, and electron affinities. The Born-Haber Cycle compares the enthalpy change of production of a specific ionic compound to the enthalpy needed to create gaseous ions from its constituent parts in order to use Hess's equation to compute lattice energies. Additional Uses for Lattice EnergiesLattice energies are more often used by scientists to assess fluoride and electron connections. The variables, in turn, help to predict the identities, elements, and characteristics of ionic compounds as well as research into the relative strengths of various ionic solids. 
Finding Lattice energyChemists often have to decide which of many ionic compounds has the greatest lattice energy. They achieve this by taking into account both the distance variable and the ion charge variable. The formula for Lattice EnergyEven though calculating exact lattice energies can be challenging, we frequently make the process simpler by applying Coulomb's Law. In accordance with this rule, the lattice energy of a certain ionic molecule may be expressed as follows: F= kQ1Q2/r^2 Q1&Q2= the relative Charges of the Constituent ions R= relative distance between them K= 2.313*10^-19 J-nm The result is expressed in Joules (J)
Next TopicLeprosy Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










