Isotonic Solution DefinitionIsotonic solutions are those that have the same tonicity. Therefore, the question of what tonicity means. Tonicity, which measures the effective osmotic pressure gradient across a semi-permeable membrane, is an estimation of the relative solute concentration across the barrier. The tonicity controls the quantity (or extent) and the direction of solvent transport across a semi-permeable barrier. It's vital to remember that the creation of an osmotic pressure gradient or the tonicity only occurs in solutes that cannot pass through a semi-permeable membrane. The phrases are not interchangeable since it is crucial to realize that iso-osmotic solutions are not always isotonic. Solutions can be divided into three categories based on the tonicity:
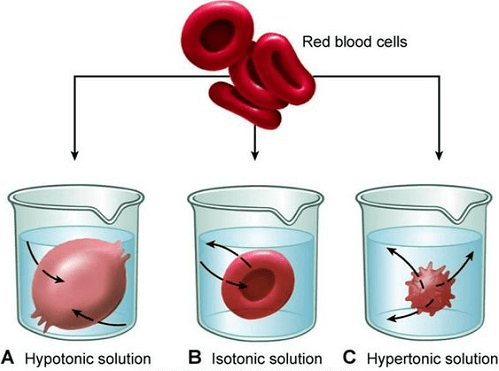
Isotonic Solution: What Is It?An equilibrium state is achieved in an isotonic solution when the solute concentration is the same on both sides of the semipermeable membrane. Due to the lack of a concentration gradient, the solvent molecules have no overall movement over a semipermeable membrane in the case of isotonic solutions. It does not imply, however, that there is no solvent movement across the membrane. In actuality, there is no movement since only the velocity of solvent transport over the membrane is constant. As a result, the solvent molecules do not move across the membrane in either a gain or a loss. The blood plasma is isotonic to a normal saline solution (9 gm/l of sodium chloride solution). An aqueous sodium chloride solution, known as a saline solution, has 9 grams of sodium chloride (0.9% w/v) in 1 liter of water. Saline is sometimes referred to as a physiologic solution because it has salt concentrations similar to those in blood. Saline solution is commonly used in medicine both topically and parenterally (i.e., by injecting it directly into the bloodstream) for purposes such as wound cleansing, sinus treatment, fluid replacement therapy, maintaining hydration, etc. since it is isotonic. The form of the red blood cells is maintained. In an isotonic saline solution, or 0.9% w/v sodium chloride solution, they neither contract nor expand. Now imagine that RBCs are dissolved in a solution that contains 2% w/v salt chloride. The solution is hypertonic because the sodium content of the RBCs is larger than the sodium chloride concentration of 2% w/v. This will cause the RBCs to contract, a process referred to as creation. In a different scenario, let's say that RBCs are dissolved in a solution that contains salt chloride at a 0.1% w/v concentration. Because the sodium content of RBCs exceeds the sodium chloride concentration of 0.1% w/v, the solution is hypotonic saline. The RBCs will expand and eventually rupture, as a result, releasing hemoglobin in the process. Hemolysis is what is happening in this instance, and it is quite harmful. Therefore, it is essential to only administer the isotonic saline solution intravenously, that is, into the bloodstream, in medicine. The intravenous method cannot provide a hyper- or hypotonic solution. It's interesting to note that intramuscular and subcutaneous injection formulations can be non-isotonic since blood cells don't immediately contact them. To prevent ocular discomfort and irritation, ophthalmic fluids should be isotonic. Additionally, isotonic with regular saline is the lacrimal fluid. While a hypertonic solution can trigger fluid to extrude from the tissue, a hypotonic solution may cause fluid to pass into the ocular tissues, congesting them. The eye of a person can still withstand 0.6% to 1.8% w/v, though. 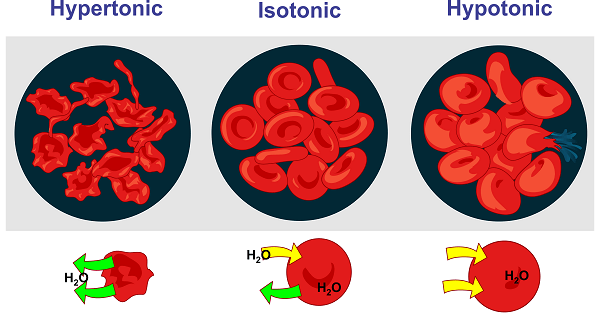
Use of Isotonic Solutions in Medicine
Application of Isotonic Solutions Outside of Medicine
Isotonic vs. Hypertonic SolutionsA solution is said to as hypertonic when its solute concentration exceeds that of the solution present over the semipermeable barrier. As a result, an osmotic pressure gradient causes the solvent to leave the cell when a cell is submerged in a hypertonic solution to achieve equal solute concentration across a membrane. Cell shrinkage is used to represent this. As a result, a cell put in a hypertonic solution experiences plasmolysis, or cell shrinkage. In this situation, the cell membrane functions as a semipermeable membrane, and the solution is referred to as "hypertonic" if the solute concentration is higher than the cytosolic concentration. Isotonic vs. Hypotonic solutionsA solution is hypotonic if its solute concentration is lower than that of the solution across the semipermeable barrier. As a result, an osmotic pressure gradient drives the solvent to migrate into the cell when submerged in a hypotonic solution to achieve equal solute concentration across a membrane. One can see this as cell swelling. The gradient difference that occurs when a cell is put in a solution with a lower solute concentration than the cytosolic concentration causes the solvent to flow into the cell, causing swelling and, ultimately, cell bursting or cytolysis. This typically occurs in animal cells because they lack a cell wall. The hard cell wall of plant cells, which have a cell membrane and a central vacuole, prevents the cell from bursting, and the vacuoles absorb extra water, which forces the cell membrane up against the cell wall. Turgor pressure is the name given to this phenomenon. ConclusionDue to equal osmotic pressures, an isotonic fluid does not encourage osmotic flow.
Next TopicKVL Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










