Solute DefinitionWhat is the Solute Definition?A material that dissolves in the solvent is referred to as a Solute. 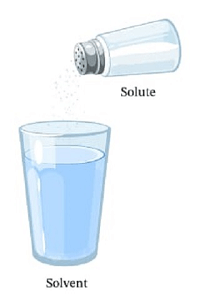
Fluid SolutionsThe presence of solvent is much greater than the amount of Solute. One common example of solutes and solvents is Salt and water. The material which gets dissolved in the solvent (namely water) is called Solute (namely Salt). Features of the Solute
Types of SolutesThe term homogenous means the mixture's components dissolve in a single phase. A heterogeneous phase means the mixture's components dissolve in a different phase. 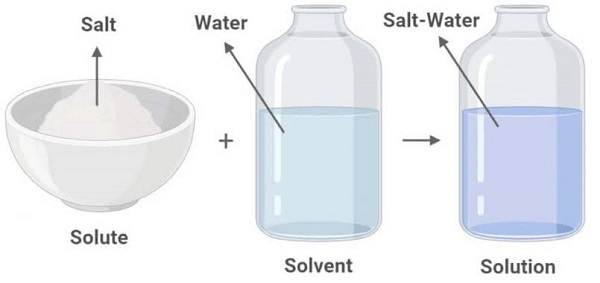
The features of the mixtures include density, temperature and concentration that can be equally distributed through the volume but only in the absence of diffusion phenomena or after their completion. The types of Solute are:
SolidA solid solute is dissolved into a solid or liquid solvent. For example Solid in solid Steel is the solution of carbon atoms in a crystalline matrix of iron atoms. Solid in Liquid: Salt (Sodium Chloride) NaCl in water Sucrose (Sugar) in Water LiquidIf the Liquid is Solute, it can be dissolved in liquids and solids. Some of the examples are: Liquid in Solid: An Amalagam is developed from Mercury in Gold. Moist solids develop when water is in solids, sugar or Salt. Hexane in Paraffin wax. The Liquid in Liquid: Alcoholic beverages are an example of solutions of ethanol in water. GaseousThe gaseous Solute can be dissolved in solid, gas and liquid solvents. We know that Nitrogen (78%) is a major constituent in the air. Here the solvent is Nitrogen, and the solution is air. Gas in Liquid: Examples of Gas in Liquid include Carbon Dioxide and Oxygen in water. Gas in Liquid: Hydrogen easily mixes in the metal (palladium). The Solute here is Palladium. Examples of Solute
Unsaturated and Saturated Solution
When a mixture dissolves solutes as much as possible at a definite temperature, it is referred to as a saturated solution. In other words, it can be defined as when no more solute can dissolve in the solvent at a particular temperature, called Saturated Solution. When the amount of Solute is not equal at the saturation level but less than that, it is referred to as Unsaturated Solution. Difference Between Solvent and Solute
Frequently Asked QuestionsQuestion: How solubility of a material change is affected by temperature? Explain with a suitable example. Answer: The highest amount of Solute that can be dissolved into a known solvent at a particular temperature is solubility. Factors that influence solubility are Pressure and Temperature. Pressure
Temperature
The solubility of a particular solute to dissolve in a particular solvent relies on the temperature. Similarly, the solubility of gases decreases with an escalation in temperature. Question: What do you consider water: A solute or solvent? Answer: The solvent is the item that determines the solutions' physical state (Liquid, solid and Gas). The Solute comes out as a product that the solvent dissolves. For example, in a solution between Sodium Chloride (NaCl) and water, the water act as a solvent and Salt as a solute. Water is popularly called " the universal solvent" because it can absorb any material compared to any liquid. Question: Define the Solution. Illustrate with examples. Answer: A solution is a mixture of two or more items that are homogenous. A solution is composed of two components, namely: a solvent and a solute. For example, Sodium Chloride is a solute in a salt solution, and water acts as a solvent.
Next TopicSolvent Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










