State Law Of Definite ProportionDid you know that a basic chemistry principle governs the food you eat, the water you drink, and the air you breathe? The set ratios of the elements that go into creating any given chemical compound are described by a law known as the Law of Definite Proportions. This law, which Joseph Louis Proust first postulated in the late 18th century, has had a significant influence on how we comprehend the natural world and the chemical processes that shape it. This article will examine the intriguing background and useful uses of the Law of Definite Proportions and show how it still influences how we think about chemistry today. Statement of the Law of Definite ProportionsA fundamental tenet of chemistry, the Law of Definite amounts, commonly referred to as the Law of Constant Composition, states that elements in a chemical compound are always present in set, definite amounts by mass. This simply indicates that, regardless of the quantity or origin of the compound, the ratio of the masses of the constituent elements in a compound remains constant. 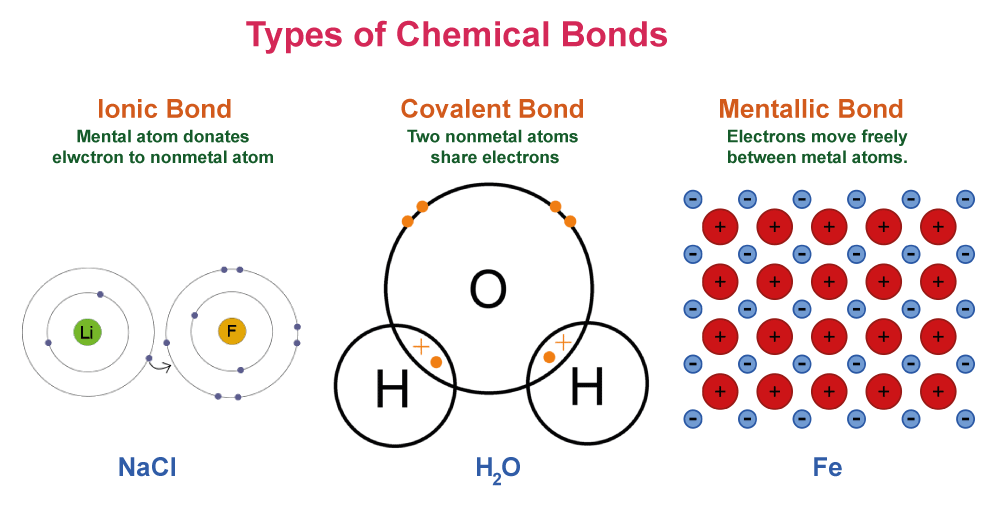
For instance, the mass ratio of hydrogen to oxygen in the compound water is fixed at 2:1. This implies that there is always 1 gramme of oxygen in water for every 2 grammes of hydrogen. For any water sample?a single drop or a gallon-sized container?this ratio holds true. Similar to this, the mass ratio of the components in carbon dioxide is fixed at 1:2 between carbon and oxygen. This implies that there are always 2 grammes of oxygen for every gramme of carbon in carbon dioxide. No matter if the carbon dioxide was created by a little flame or a volcanic explosion, this ratio is true for all samples of the gas. The Law of Definite Proportions is the foundation of several practical applications in chemistry and beyond. It aids scientists in the identification of compounds and the determination of their properties. History and DiscoveryFrench scientist Joseph Louis Proust first proposed the Law of Definite Proportions in the late 18th century. Proust performed multiple experiments on chemical compounds and found that no matter how much of the compound he used, the mass ratios of the elements remained constant. As a result, he developed the Law of Definite Proportions, which stipulated that in any given chemical composition, the elements are always present in set, definite proportions by mass. The finding made by Proust was a significant development in the science of chemistry since it laid the groundwork for our knowledge of the structure and characteristics of chemical compounds. Before his research, there was a great deal of disagreement among chemists over the question of whether compounds could have different compositions or whether they always contained set ratios of their component elements. It was settled by Proust's experiments and observations, which made the Law of Definite Proportions a foundation of chemistry. Some More ExamplesAll chemical compounds are subject to the Law of Definite Proportions, or the Law of Constant Composition, and there are innumerable instances of it in action. Here are a few more instances:
ApplicationsThis law has several practical applications in chemistry. For example, it allows chemists to determine the chemical formula of a compound based on the masses of the elements it contains. This is because the law of definite proportion ensures that the ratio of the masses of the elements in the compound is always the same, regardless of the amount of the compound present. The law of definite proportion also plays a crucial role in determining the purity of a substance. By analyzing the ratios of the masses of the elements in a sample, chemists can determine the degree of purity of the substance. If the ratios are consistent with the expected ratios for a pure substance, then the sample is likely to be pure. Additionally, the law of definite proportion has practical applications in industries such as pharmaceuticals and agriculture, where precise composition of chemical compounds is crucial for their effectiveness. This law ensures that correct ratios of elements are present in these compounds, which is necessary for their desired properties and effects. Overall, the law of definite proportion is a fundamental principle in chemistry that has many practical applications in various fields, including determining chemical formulas, assessing purity, and ensuring the effectiveness of chemical compounds. Relationship to Other LawsThe law of definite proportion is closely related to other laws of chemistry, as it helps to explain and support these laws. One such law is law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction. Law of definite proportion supports this law by ensuring that total mass of the elements in a compound remains constant, even as compound undergoes chemical reactions. The law of multiple proportions, which asserts that the mass ratios of one element that combine with a fixed mass of another element can be stated in tiny whole numbers when two elements create multiple compounds, is connected to the law of definite proportion. Due to the constant ratios of the elements in a compound, it is possible to describe the ratios of the elements in different compounds in small whole numbers, which is supported by the law of definite proportion. The law of definite proportion is related to atomic theory, which states that all matter is composed of atoms. Law of definite proportion supports this theory by showing that proportions of elements in a compound are fixed because they are determined by atomic structure of the elements. Overall, the law of definite proportion is an important law of chemistry that is closely related to other fundamental laws, such as the law of conservation of mass, law of multiple proportions, and the atomic theory. Together, these laws help to explain the behavior of matter and the nature of chemical reactions. Some Questions Based On Law of Definite ProportionQues 1: 2.5 g of hydrogen and 32.5 g of oxygen make up a compound. Determine hydrogen to oxygen mass ratio in this molecule. Answer: By dividing the mass of hydrogen by the mass of oxygen, one can determine mass ratio of hydrogen to oxygen. Mass of hydrogen = 2.5 g Mass of oxygen = 32.5 g Mass ratio of hydrogen to oxygen = Mass of hydrogen/Mass of oxygen = 2.5/32.5 = 0.077 Therefore, the mass ratio of hydrogen to oxygen in this compound is 0.077. Ques 2: There are 34.6 g of copper and 16.0 g of sulphur in a sample of copper sulphide. Determine the copper to sulphur mass ratio in this combination. Answer: Mass ratio of copper to sulfur can be calculated by dividing mass of copper by the mass of sulfur. Mass of copper = 34.6 g Mass of sulfur = 16.0 g Mass ratio of copper to sulfur = Mass of copper/Mass of sulfur = 34.6/16.0 = 2.16 Therefore, mass ratio of copper to sulfur in this compound is 2.16. Ques 3: 24.0 g of carbon and 16.0 g of oxygen make up a compound. What is carbon to oxygen mass ratio in this compound? Answer: By dividing mass of carbon by the mass of oxygen, the mass ratio of carbon to oxygen can be calculated. Mass of carbon = 24.0 g Mass of oxygen = 16.0 g Mass ratio of carbon to oxygen = Mass of carbon/Mass of oxygen = 24.0/16.0 = 1.5 Therefore, mass ratio of carbon to oxygen in this compound is 1.5. ConclusionThe Law of Definite Proportions an essential tenet of chemistry, which controls the predetermined ratios of the constituent parts of chemical compounds. This law has significantly impacted our understanding of the natural world and the chemical processes that shape it. The Law of Definite Proportions is a key factor in establishing a substance's purity since it enables scientists to calculate the chemical formula of a compound based on the masses of the elements it contains. It has useful uses in fields like agriculture and medicines where the precise chemical composition of molecules is essential to their efficiency. Other chemistry rules are strongly related to the Law of Definite Proportions, which both supports and explains them. Overall, this law of definite proportions is a pillar of chemistry that has aided in our understanding of composition and properties of chemical compounds and continues to be useful in a variety of contexts
Next TopicBronchitis Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










