Homogeneous Definition
A material is homogenous if its qualities and composition are constant throughout all its samples. Latin's word for homogeneous means of the same sort. It is referred to as heterogeneous when a material is not homogenous. Homogeneous types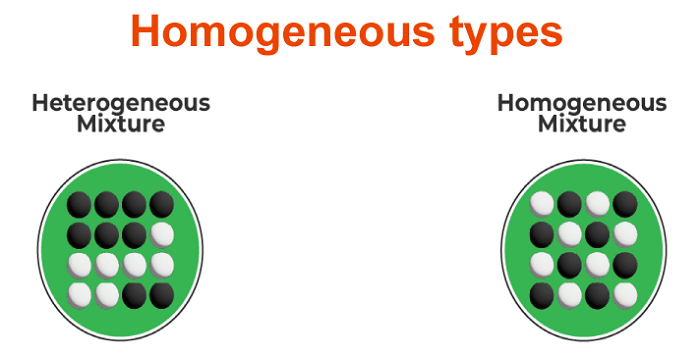
A homogenous mixture has the same proportions of constituent parts throughout a particular sample, whether solid, liquid, or gaseous. Its makeup is constant throughout. In a homogenous combination, just one phase of matter is visible. Homogenous solution causes
Solutions with a homogenous composition and set of characteristics are said to be homogeneous. For instance, a cup of coffee, perfume, cough medication, a salt- or sugar-in-water solution, etc. Heterogeneous solutions have a non-uniform composition and a variety of features throughout. Homogeneous population
The term "homogeneous population" refers to a group of individuals who make up a tiny or nonexistent portion of a population. For illustration, in a community living on an island where there is no inward migration of people, all of the individuals will be homogeneous for a certain attribute like disease immunity. Homogenous examplesThey are all the same in terms of look and chemical creation. Examples are
About term homogeneousIn section 9 of his 1726 article De integrationists aequationum differentialium, John Bernoulli used the term "homogeneous" for differential equations for the first time. (On the integration of differential equations). Homogeneous's MeanThe homogeneous' mean It comes from the Greek homogenous, which is derived from homo-, "same," and génos, "kind," and means "of the same type." Homogeneous means things that are fundamentally uniform or similar throughout-without any substantial variation-in every context in which it is used. Homogenous combination of water
A homogenous combination is a water. A homogeneous mixture has a uniform appearance; you cannot distinguish the components from one another while looking at it. Hydrogen and oxygen cannot be distinguished from one another in water. It is a homogenous combination as a result. Some Illustrations About Homogeneous
Illustration 1: Homogeneous chemical elements are possible. Examples of homogeneous elements include gold in an ingot, mercury in a gauge, and nitrogen in a balloon. Illustration 2: Homogeneous compounds are possible. Homogeneous materials include: Bubbles of carbon dioxide. Bottled water. Plastic forms an electrical plug. Illustration 3: Homogeneous mixtures are possible. Saltwater in a bottle, the air in a balloon, and brass ingots illustrate homogenous mixtures. Illustration 4: Not all chemical components must be homogenous. A sample of tin, for illustration, that tin pest present is not a homogenous element. Illustration 5: Compounds don't have to be homogeneous. Water that has partially frozen and become slushy is not a homogenous substance. Illustration 6: It's not necessary for mixtures to be homogenous. Sand and water are not combined evenly in a spoonful. Illustration 7: A homogeneous catalyst is present in the same phase as the reactants, often a liquid phase for some reactions and occasionally a gas phase for others. Illustration 8: When reactants and products are all in the same phase, a balance is said to be homogeneous. Questions and answers about HomogeneousQuestion 1: What is another illustration of homogeneous? Answer: Most alloys, air, saline solution, and bitumen are homogenous mixes. Question 2: Has milk been homogenized? Answer: But, as we know, fat and water components cannot be combined to make a solution. Milk is a heterogeneous combination since it has two immiscible liquid phases. Question 3: Whose nature is homogeneous? Answer: In nature, ice and air are identical. Ice and air are homogenous materials because their mass consistency is constant. Question 4: How homogenous is salt? Answer: One can't distinguish the components of a homogenous mixture since they are scattered equally across the volume-a salt solution, as an illustration. A salt solution is homogenous as a result. As a result, the salt solution is an impure, homogenous mixture. Question 5: Is ocean water homogeneous? Answer: Seawater combines salts, water, and several other suspended contaminants. It also includes a variety of liquid molecules in addition to these. Seawater is categorized as a homogenous mixture since it has a variety of dissolved gases.
Next TopicHomogeneous Mixture Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










