Definition of Atomic NumberChemists are able to predict chemical elements' characteristics and behaviour in chemical compounds by comprehending the underlying qualities that define them. This information enables the discovery of novel chemical forms as well as our grasp of the basic principles governing nature's world. One of these fundamentals is the volume of atoms in each element. Along with their location in the periodic table, the number of electrons that are present and interact directly with other molecules is also revealed. Prior to being able to understand the concepts of atomic number and mass number of elements, one must first grasp the fundamentals of atoms. A planetary system and an atom's structure have both been likened in the past. The centre of the atom is a positively charged atomic nucleus, which contains almost all of the mass of the atom. Around the exterior of the nucleus, electrons with negative charges are moving. They are drawn in their direction by the nucleus' electrostatic forces. The electrons, particularly those in the valence shell, which is farthest from the nucleus, are in charge of many atom: level characteristics. The atomic number of a nucleus, which always equals the number of electrons in its orbit, is the number of protons it contains (in a nonionized atom). Since all atoms with the same atomic number: the number of protons: are atoms of the same element. 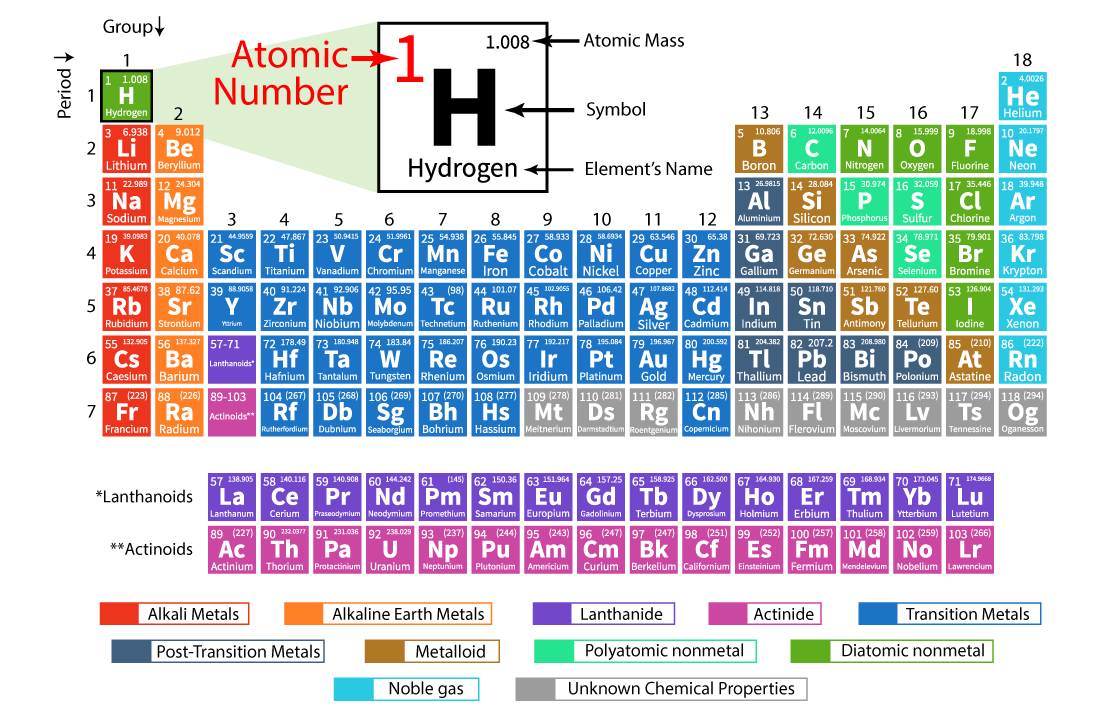
Each element has an atomic number, which is one of its fundamental properties, but it does not appear in the name or symbol of the element; rather, it is typically written as Z (for 1s) followed by its mass number, which is the sum of all protons plus neutrons in the nucleus of that specific element (for instance: Z=1+1=2). Each atom's basic characteristic, which includes its shape and size, dictates how it behaves chemically. The nuclei of atoms with more protons tend to be larger than those with fewer protons, in contrast. In the case of elements with 17 or 18 neutrons, this rule is broken (elements with this configuration are referred to as "island" nuclei). The atomic number, abbreviated Z, which indicates how many protons are in an atom's nucleus, is used to organise the elements in the periodic table. Because every chemical has a unique number of protons, an element can be identified by its atomic number. For instance, the digits 6 and 92 are always stand for carbon and uranium, respectively. Atomic number and mass number, which is the total of an atom's protons and neutrons, are two separate ideas. In certain cases, the atomic number is specified in descriptions of specific atoms, and in other cases, it is deduced from the chemical name (if the chemical name is listed as nitrogen, the atomic number must be 7). 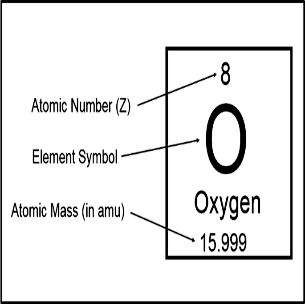
The number of protons in a specific atom is represented by its atomic number. Each element has a fundamental characteristic that governs its chemical properties, including its shape and size. Because it relates to a particular particle called an electron, the atomic number is also known as the proton number. The number of protons in a specific atom is its atomic number. Each element's basic characteristics, including shape and size, are what determine its chemical properties. Because it relates to a particular particle called an electron, the atomic number is also known as the proton number. History of periodic tableMendeleev grouped the 63 elements in a vertical column named groups and horizontal rows called periods because it was thought that the properties of elements were periodic functions of their atomic masses. The placements of various elements, rare earth metals, and isotopes, however, were unable to be explained by this classification system. It was therefore turned down. The "Atomic number" was a new characteristic of elements that Henry Mosley introduced in 1923. He thought that the atomic number of an element was a more fundamental characteristic than the atomic mass. Dobereiner's Triads states that when atoms are arranged in ascending order of atomic mass, the middle element's atomic mass is nearly equal to the arithmetic mean of the other two, and its attributes are midway between those of the other two. Mendeleev studied the characteristics of all 63 elements and their compounds that were known at the time. He found that elements with similar properties frequently appear when he grouped the elements in ascending order of atomic mass. Mendeleev's Periodic Law, which sums up his discovery, was first published in 1869. Terms related to atomic number
Next TopicDefinition of Water Pollution
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










