Lipids DefinitionBy definition of Lipids, "Lipids are organic compounds with hydrogen, carbon, and oxygen atoms that serve as the structural and metabolic foundation of living cells." In general, they are often referred to as fats and oils. Lipids: What do they mean?These organic substances are made up of non-polar molecules that can only dissolve in non-polar solvents and are inert in water due to water's polar nature. These compounds can be produced in the liver of the human body and are also present in foods like oil, butter, whole milk, cheese, cooked foods, and some types of red meat. 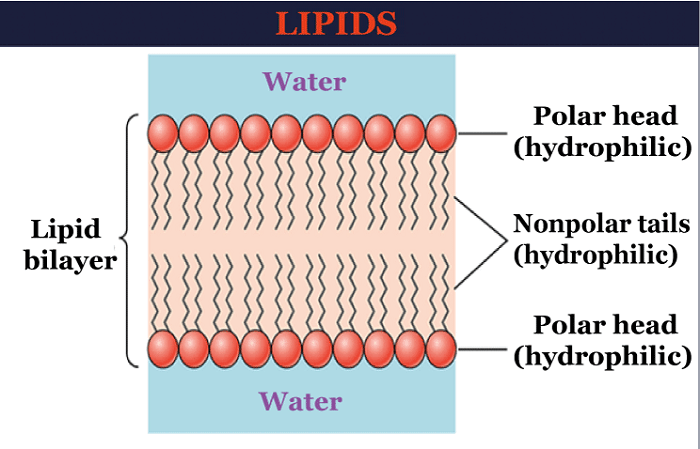
Let's take a closer look at the lipid structure, characteristics, kinds, and lipid categorization. Lipids' QualitiesFats and oils make up the class of chemical substances known as lipids. These compounds produce a lot of energy and are in charge of several bodily processes. The following list includes some important characteristics of lipids:
Lipid CompositionLipids are fatty acid polymers with long, non-polar hydrocarbon chains and a relatively tiny polar area holding oxygen. The following picture consists of lipid structures, showing saturated and unsaturated lipids: 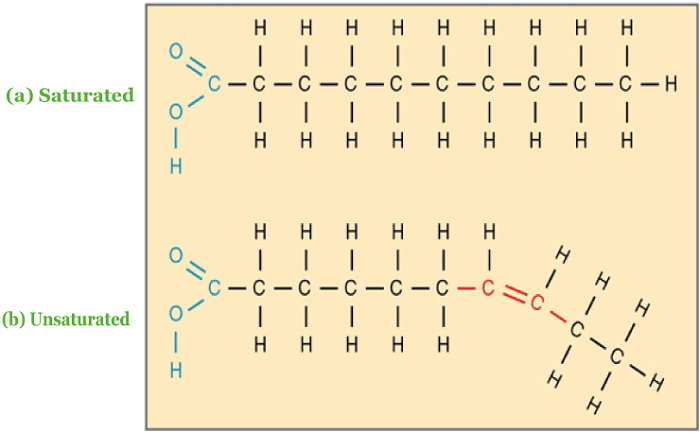
Lipid ClassificationThere are two major categories into which lipids fall, such as: Non-saponifiable Lipids Hydrolysis cannot break down a non-saponifiable lipid into smaller units, so they are called non-saponifiable lipids. Lipids that cannot be saponified include prostaglandins, cholesterol, etc. Saponifiable LipidsWax, triglycerides, sphingolipids, and phospholipids are examples of saponifiable lipids, which can be hydrolyzed in the presence of a base, acid, or enzymes because they contain one or more ester groups. These groups can also be separated into polar and non-polar lipids. The key building blocks of all these lipids are fatty acids. Triglycerides, a type of non-polar fatty acids, are used as fuel and energy storage. Membranes use polar lipids that could create a barrier with an exterior aqueous environment. Sphingolipids and glycerophospholipids are two types of polar lipids. Different LipidsThere are many distinct lipid types that are vital to living, including fatty acids, triglycerides, glycerophospholipids, sphingolipids, and steroids. However, they all fall under two main groups of lipids. Generally speaking, lipids can be broadly divided into the following two forms: Simple LipidsSimple lipids are defined as fatty acid esters with different alcohols. Esters of fatty acids in combination with glycerol make up fats. Oil is the one distinct form of fat but in a liquid form. In contrast, higher molecular weight monohydric alcohol esters of fatty acids are classified as waxes. Complex LipidsComplex lipids are defined as fatty acid esters that may contain other groups in addition to alcohol or fatty acids. Phospholipids are lipids that additionally incorporate the phosphate group along with fatty acids and alcohol. They commonly have bases that contain nitrogen as well as other substituents, such as sphingosine for sphingophospholipids and glycerol for glycerophospholipids. A fatty acid, sphingosine, and glucose are all components of glycolipids (also known as glycosphingolipids). Additionally, lipids like amino lipids and sulfolipids are classified as other complex lipids. This classification may also include lipoproteins. Below, a more detailed description of some of the other different lipid types is provided: Precursor and Derived LipidsThese include lipid-soluble vitamins, hormones, glycerol, steroids, other alcohols, fatty aldehydes, and ketone bodies, as well as hydrocarbons. Acylglycerols (glycerides), cholesterol, and cholesteryl esters are referred to as neutral lipids because they are uncharged. Simple and complicated lipids are hydrolyzed to create these specific molecules or compounds. Fatty Acids In general, fatty acids have lengthy aliphatic ends (long chains) and are either saturated or unsaturated carboxylic acids (also known as organic acids).
Role of FatsIn our bodies, fats perform a number of important functions. The following list includes some of the crucial functions of fats:
Examples of LipidsLipids come in a variety of forms. Butter, ghee, veggie oil, cheese, cholesterol and other steroids, waxes, phospholipids, and fat-soluble micronutrients are a few examples of lipids. All of these substances share similar characteristics such as being inert (or insoluble) in water while being soluble in organic solvents. WaxesLong-alcohols and long-chain carboxylic acids combine to make waxes, which are "esters" (an organic compound manufactured by substituting an alkyl or other organic group for the hydrogen in an acid-containing molecule). The world is practically covered with wax, which means that waxes are easily available and identifiable. Waxy coatings are present on the seeds and foliage of many plants, protecting them from dehydration and tiny animals. The same coverings that serve as water repellants are present in some animals' fur and in birds' plumage as well. Water resistance and durability are two notable properties carnauba wax is famous for, making it a valuable choice for car wax products. PhospholipidsPhospholipids, or phosphoacylglycerols, make up the majority of the molecules in membranes. 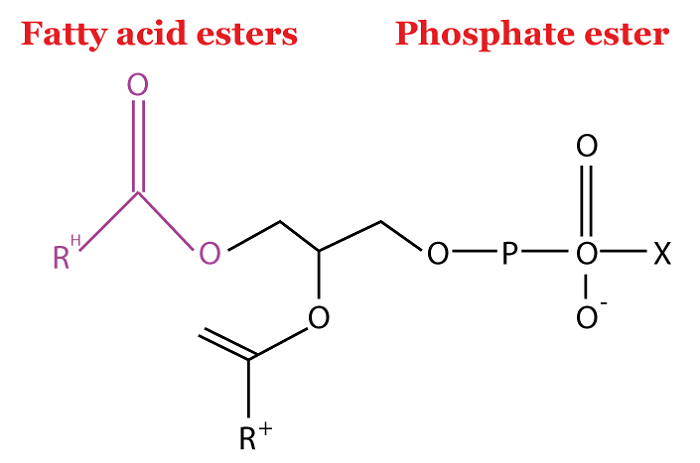
Triacylglycerols and phosphoacylglycerols are identical; however, phosphatidic acid is produced when the terminal OH group of the phosphoacylglycerol is esterified with phosphoric acid rather than a fatty acid. Because of this, the formation of phosphatidic acid occurs. Because phosphoacylglycerols are lipids with a phosphate group, they are known as phospholipids. SteroidsHormones, which are essentially organic substances produced in glands and delivered by the bloodstream to different tissues, are chemical signals that our bodies have that can be used to either initiate or obstruct specific processes. Steroids are also classified as a distinct form of hormones. The tetracyclic skeleton of steroids, which is made up of three fused six-membered rings and one five-membered ring, is usually used to identify this type of hormone. The four rings are denoted by the letters A, B, C, and D, as seen in the blue tint, while the numerals in red denote the carbons. CholesterolOnly meals derived from animals contain cholesterol, a wax-like material. The various kinds of cholesterol present in blood cells include triglycerides, LDL, HDL, and VLDL. The cell wall also contains cholesterol, an essential fat. Since cholesterol is a sterol, it is a compound of a steroid and an alcoholic beverage. In the human body, the liver is where cholesterol is created. All living cells produce these substances through biosynthesis, and they are necessary for the cell membrane's morphological integrity. The cholesterol's steroid ring structure in the cell membrane offers a stiff hydrophobic structure that contributes to the cell membrane's increased rigidity. The cell barrier wouldn't function properly without cholesterol. The reproductive hormones estradiol and testosterone, as well as other steroids like cortisone and vitamin D, are all produced from this essential component of cell membranes. It also serves as the starting point for the production of other steroids.
Next TopicAbstract Noun Definition and Examples
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










