Definition Of ElectronElectrons are found in electron orbitals or shells outside the atomic nucleus and are subatomic particles with a negative charge. Through tests involving cathode rays, J.J. Thomson made their initial discovery in 1897. As the catalysts of chemical processes and the building blocks of chemical bonds, electrons are essential to both chemistry and physics. Considering that they are the charge carriers that pass through conductive materials, allowing for the transmission of electrical energy, they also play a crucial part in electricity. 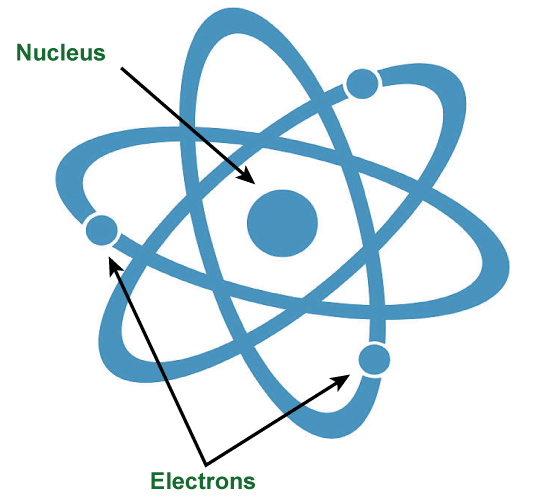
The structure and quantity of electrons in an atom dictate the specific energy levels or orbitals that the electrons inhabit. There can be up to two electrons in the first electron shell, and there can be up to eight electrons in each of the second and third electron shells. An atom's reactivity and chemical characteristics are governed by the number of electrons in its outermost shell. According to the experiment, electrons may act both like waves and like particles because they display wave-particle duality. The wave function, a mathematical function that calculates the likelihood that an electron would be found at a specific position, describes this duality. In addition, the Pauli exclusion principle applies to electrons, stipulating that no two atom electrons may have the same set of quantum numbers. This theory explains the stability of matter and the creation of chemical bonds. Electrons are essential in numerous technical applications, including electronics, telecommunications, computers, and their role in atoms. They enable contemporary technology by being employed in transistors, microchips, and other electronic components. History Of The Study Of ElectronsScientists like J.J. Thomson and Robert Millikan did ground-breaking work in the late 19th century that gave rise to the idea that electrons are subatomic particles. They performed tests to determine the charge and mass of these minute particles and offered proof that electrons existed. Before the discovery of electrons, the dominant idea was that matter was composed of only three fundamental particles-protons, neutrons and electrons-and that atoms were indestructible. However, Thomson discovered negatively charged particles that he dubbed "corpuscles" and are today known as electrons due to his cathode ray tube studies. Chemistry and physics both benefited greatly from the discovery of electrons. It gave an explanation for a number of previously inexplicable phenomena, including how electricity and magnetism behave. The characteristics of electrons and how they behave in atoms were continuing to be researched in the years that followed. Early in the 20th century, Niels Bohr put out an atom model that included electrons in distinct energy levels or "shells" surrounding the nucleus. Numerous significant findings in the field of quantum mechanics were made as a result of the model, which offered a framework for comprehending the behaviour of electrons in atoms. Even now, physics and chemistry researchers are still heavily investing in the study of electrons and their characteristics, as many processes are justified based on the basis of the properties of electrons. Numerous real-world uses for electrons have been made possible by their discovery and comprehension, including the advancement of electronics and contemporary technology in all of their potential capacity. The history and identification of electrons felicitated the study of natural phenomena. The branch is ever-growing in terms of properties. Structure And Types Of ElectronsAn atom's outermost region contains electrons, subatomic particles with a negative charge. In many chemical and physical processes, such as the creation of chemical bonds and electricity, they are essential. A rather basic structure governs an electron. It consists of a single-point particle that is negatively charged and has a mass that is around 1/1836 that of a proton. The energy level, or how far an electron is from the nucleus, is used to characterize electrons. Valence and core electrons are the two primary subtypes of electrons. Chemical bonds are formed by valence electrons, which are found at an atom's highest energy level. The electrons that take part in chemical reactions are those that determine an atom's reactivity. Contrarily, core electrons, which are found at the atom's lower energy levels, do not participate in chemical bonding. Bonding electrons and non-bonding electrons are additional categories into which valence electrons can be further subdivided. Chemical bonds are created by the sharing or transfer of bonding electrons, which can occur between atoms. Non-bonding electrons, sometimes referred to as lone pair electrons, reside in unshared pairs and are not engaged in chemical bonds. The term "free electron" is also used to describe another kind of electron. Free electrons can move freely in a substance since they are not attached to any one atom. They are crucial to the operation of electronic devices because they control the passage of electrical current in conductive materials. 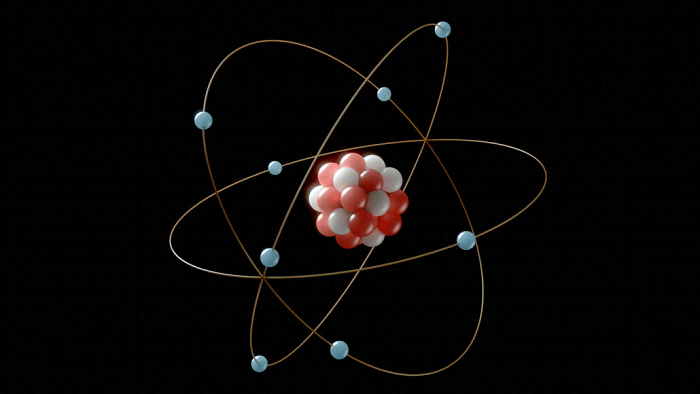
Properties Of Electrons
Bonds By ElectronsMolecules are created when atoms unite through various kinds of chemical bonds. Covalent, ionic, and metallic connections are the three main kinds of chemical bonding.
Practical Applicational StudyAtoms contain negatively charged electrons, which are basic particles. They are essential to many real-world apps that we use every day. Here are a few examples of how electrons are used in everyday life:
Behavioral Phenomenon Of ElectronsChemistry's "electron configuration" notion specifies how electrons are positioned in an atom's energy levels or orbitals. The behavior of electrons, which are negatively charged particles that surround the atomic nucleus, forms the basis for the electrical configuration of an atom. Around an atom's nucleus, electrons are scattered in several energy levels, each of which has a specific number of sublevels or orbitals. The number of electrons that each of these orbitals can accommodate affects the atom's electrical structure. The principal quantum number (n) refers to the energy level. The sublevel letter (s, p, d, or f), which refers to the shape of the orbital, and the superscript number, which refers to the number of electrons in the orbital, is used to represent the electronic configuration of an atom. Carbon, for instance, has an electrical configuration of 1s2 2s2 2p2, which indicates that it contains two electrons in its first energy level, two electrons in its second energy level's s orbital, and two electrons in its second energy level's p orbital. Understanding atoms' physical, chemical, and bonding characteristics and their reactivity, uses the electronic configuration idea. How an atom interacts with other atoms and molecules depends on how many and how organized the electrons are in its orbitals. The behavior of electrons is the basis for a number of chemical processes. Here are a few illustrations:
Numerous chemical phenomena, including chemical bonding, conductivity, the photoelectric effect, redox reactions, and spectroscopy, are greatly influenced by the behavior of electrons. A firm understanding of the electronic configuration idea and the behavior of electrons in atoms and molecules is necessary to comprehend these events.
Next TopicHypoxemia Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










