Polymerization Definition
What is Polymer?
A polymer is a big molecule made up of smaller parts, called monomers, that are connected together chemically. This process is called polymerization. Polymers can either occur naturally, such as DNA and proteins or be man-made, like plastics and synthetic fibers.
Polymers can have many different physical and chemical properties, which depend on the type of monomer used, how many are used, and how they are arranged in the polymer. Some physical properties of polymers include how flexible or strong they are, how elastic they are, and how well they can handle high temperatures. Some chemical properties of polymers include how they react with other chemicals, how easily they dissolve, and how resistant they are to break down.
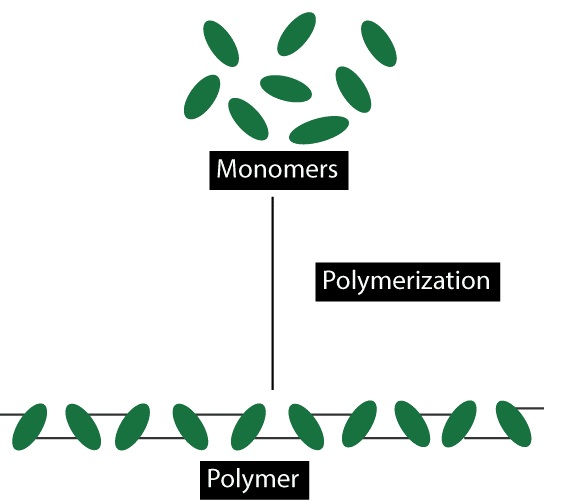
Polymers are used in many different industries and everyday life. They can be used to make things like plastics, adhesives, coatings, fibers, and films. In medicine, polymers can be used to make things like drug delivery systems, structures for tissue engineering, and materials for medical devices.
The study of polymers is called polymer science or polymer chemistry. Polymer scientists and chemists work to create new polymers with unique properties and to understand how polymers behave in different situations.
Polymerization Definition
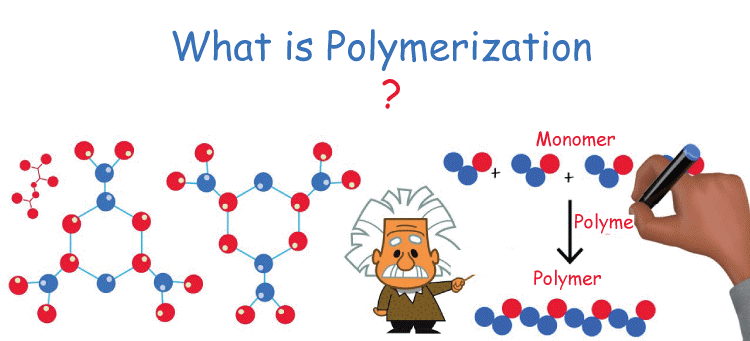
Polymerization is a chemical reaction in which small molecules, called monomers, combine to form a larger molecule, called a polymer. During this process, the monomers link together through covalent bonds, resulting in a chain-like structure. Polymerization can occur through various mechanisms, including addition polymerization, condensation polymerization, and radical polymerization.
The resulting polymer can have a range of physical and chemical properties that are dependent on the monomer composition and the reaction conditions. Polymers are widely used in various industrial and commercial applications, including plastics, fibers, adhesives, and coatings.
Properties of Polymer
Polymers can have a wide range of properties, depending on their monomer composition, degree of polymerization, and molecular structure. Some of the properties that are commonly associated with polymers include:
- Mechanical Properties: Polymers can have a range of mechanical properties, such as stiffness, flexibility, elasticity, and toughness. For example, some polymers are rigid and brittle, while others are flexible and elastic.
- Thermal Properties: Polymers can have different thermal properties, such as melting point, glass transition temperature, and thermal conductivity. Some polymers are resistant to heat, while others can melt or decompose at high temperatures.
- Chemical Properties: Polymers can have different chemical properties, such as reactivity, solubility, and resistance to degradation. Some polymers are inert and do not react with other chemicals, while others are highly reactive and can undergo chemical reactions with other substances.
- Electrical Properties: Polymers can have electrical properties, such as conductivity, dielectric constant, and insulation properties. Some polymers are good electrical conductors, while others are insulators.
- Optical Properties: Polymers can have optical properties, such as transparency, refractive index, and light scattering. Some polymers are transparent, while others are opaque or translucent.
- Environmental Properties: Polymers can have environmental properties, such as resistance to UV radiation, moisture, and chemicals. Some polymers are resistant to environmental factors, while others can degrade or deteriorate over time.
These properties can be adjusted by changing the monomer composition, reaction conditions, and post-polymerization treatments, making polymers a versatile and useful class of materials.
Scientists can adjust these properties by changing the type of monomers used, reaction conditions, and post-polymerization treatments. Polymers are useful because they can be tailored to have specific properties for different applications, such as making plastic bottles, fibers for clothing, or medical devices.
Classification of Polymerization
Based on the Mode of Polymerization
Various mechanisms through which Polymerization can occur-
- Addition Polymerization: Monomers containing a double bond are activated by a catalyst and undergo a chain reaction, leading to the formation of a polymer chain. This type of polymerization is used to produce a wide range of materials, including plastics and synthetic rubbers. Addition polymerization is a way of making a polymer by adding monomers together without any byproducts. This means that the polymer chain is made up of the same repeating monomer unit.
The process of addition polymerization happens through a free-radical mechanism, which starts when a free radical initiator, like a peroxide, creates a free radical. This free radical then reacts with a monomer, which causes it to become a free radical too, and bond with another monomer. This process keeps going until a long chain of polymer is formed.
Scientists can control the properties of additional polymers by adjusting the polymerization conditions. For instance, they can control the molecular weight of the polymer by changing the concentration of the monomer and initiator. They can also control the tacticity, which is the way the monomers are arranged in the polymer chain, by using different types of monomers or catalysts.
In addition, polymers have a lot of uses in industry, such as in the production of plastics, synthetic rubbers, and coatings. Because they're very strong and durable, they're useful for many products, from car parts to food packaging. Addition polymerization is a well-established process in polymer chemistry and is always being developed to create new and innovative materials.
- Condensation Polymerization: On the other hand, is a process used to make different types of polymers by combining two or more monomers that have functional groups that can react with each other. This reaction results in the formation of covalent bonds, and small molecules like water, alcohol, or hydrogen chloride are released as byproducts. The reaction continues until all the monomers are consumed, and a high molecular weight polymer is formed.
Condensation polymers have some important properties, such as being strong, durable, and resistant to heat, chemicals, and moisture. They are used in many applications, including fibers for clothing, packaging materials, and engineering plastics.
Some common examples of condensation polymers include polyester, which is used in fabrics, films, and packaging; polyamide, which is used in carpets, clothing, and car parts; and polycarbonate, which is used in optical lenses, electronic components, and medical devices.
Condensation polymerization is different from addition polymerization because it involves the loss of small molecules, whereas addition polymerization involves the opening of double or triple bonds present in monomers without the loss of any molecule.
In summary, condensation polymerization is a useful process that allows the production of a wide range of polymers with different properties that can be used in many different applications.
- Radical Polymerization: A free radical is generated and initiates the polymerization reaction. This type of polymerization is used to produce materials such as polystyrene and polyvinyl chloride. Radical polymerization is a process that uses free radicals to create polymers. To do this, initiators are used to create free radicals under certain conditions, like heat, light, or chemicals.
There are three main stages to the reaction: initiation, propagation, and termination. In initiation, the initiator creates free radicals that react with the monomers, creating new radicals. In propagation, these new radicals react with additional monomer molecules, creating long chains of polymers. Termination occurs when two radicals combine to stop the chain growth.
The benefit of radical polymerization is that it can produce a wide range of molecular weights and structures, making it useful for many industrial applications like plastics, synthetic rubber, and adhesives. However, there are some challenges to the process like controlling the molecular weight and distribution of the polymer chains, which can negatively impact the final product's properties.
Researchers have developed new techniques like controlled radical polymerization and reversible addition-fragmentation chain transfer to better control the process and produce polymers with improved properties and performance.
Overall, radical polymerization is an essential process for producing high-performance polymers for various applications, and with new techniques, it continues to be an area of research and development in materials science.
Based on Source
Polymerization can be divided into two categories based on their source:
- Natural Polymerization: Natural polymerization occurs naturally in living organisms and involves the formation of biopolymers like proteins, DNA, and carbohydrates. These polymers are formed through complex biological processes and have essential roles in the structure and function of living organisms.
- Synthetic Polymerization: On the other hand, is a human-made process that creates a variety of synthetic polymers using chemical reactions. This process allows the production of a wide range of synthetic polymers with specific properties for use in various industries. Synthetic polymers include plastics, synthetic fibers, and rubber.
Based on the Structure of Polymers
- Linear Polymers- Linear polymers are long chains of monomers that are linked together in a straight line, without any branching. They have a simple and linear structure, which can be characterized by their high molecular weight and low density.
Linear polymers can have a wide range of properties, depending on the type of monomers used and the degree of polymerization. They can be either crystalline or amorphous and can be either soluble or insoluble in solvents. Linear polymers are commonly used in various applications, such as packaging materials, fibers, films, and coatings.
- Branched Polymers- Branched polymers are a type of polymer in which some of the polymer chains have side chains branching off from the main polymer backbone. These side chains can be short or long and can have their own branches, resulting in a complex, tree-like structure. Branched polymers can have different properties compared to linear polymers, such as a lower viscosity and improved elasticity, due to the increased number of chain ends and entanglements.
The degree of branching, or the number of side chains per unit length of the main chain, can also affect the physical and chemical properties of the polymer. Branched polymers have a wide range of applications in industry and everyday life, such as in adhesives, coatings, and personal care products.
- Network or Cross-Linked Polymers- Network or cross-linked polymers are polymers in which the individual polymer chains are connected to each other by covalent bonds, forming a three-dimensional network. The network structure gives these polymers high strength, rigidity, and toughness, as well as resistance to swelling and solvents.
Cross-linked polymers can be formed by polymerizing a monomer that contains three or more functional groups, or by cross-linking linear or branched polymers after they have been formed. These polymers are used in a variety of applications, such as in adhesives, coatings, and as materials for contact lenses, dental fillings, and artificial organs.
Based on Focus on Molecules
- Elastomers- Elastomers are a type of polymer that is highly elastic and deformable, with the ability to return to their original shape after being stretched or deformed. They are typically amorphous and have a high molecular weight, which contributes to their ability to stretch and recoil.
Elastomers are often used in applications where flexibility and resilience are important, such as in tires, seals, and gaskets. They can also be used as coatings, adhesives, and in other specialized applications, such as biomedical devices. Some common examples of elastomers include natural rubber, synthetic rubber, and silicone rubber
Elastomers are typically formed through addition polymerization, which involves the reaction of monomers with unsaturated bonds to form a polymer chain. The resulting polymer chains are then cross-linked to create a three-dimensional network structure that gives elastomers their unique properties.
- Fibres- Fibers are a type of polymer that has a long, thin shape. They can be natural or synthetic and are commonly used to make textiles and fabrics. Natural fibers, such as cotton, wool, and silk, come from plants and animals, while synthetic fibers, such as polyester and nylon, are made from chemicals.
Fibers can be classified based on their source, chemical composition, and physical properties.
Natural fibers are typically composed of cellulose or protein molecules, while synthetic fibers are made from polymers such as polyester, nylon, and acrylic.
Fibers can also be categorized based on their physical properties, such as strength, flexibility, and absorbency. For example, cotton fibers are known for their softness and absorbency, while nylon fibers are strong and durable.
Fibers can be spun into yarns, which can then be woven or knitted into fabrics. The properties of the fibers determine the characteristics of the resulting fabric, such as its strength, texture, and drape. Different types of fibers can also be blended together to create fabrics with unique properties.
Overall, fibers are a versatile material used in a wide range of applications, from clothing and textiles to industrial materials and composites.
- Thermoplastic Polymers- Thermoplastic polymers are a type of polymer that becomes soft and pliable when heated and solidify when cooled. They can be melted and re-molded multiple times without undergoing any significant chemical change. This behavior is in contrast to thermosetting polymers, which harden irreversibly when heated and do not melt upon reheating.
Thermoplastic polymers are widely used in various applications due to their excellent properties, such as high strength, durability, flexibility, and low density. They can be processed using a variety of techniques, such as injection molding, extrusion, blow molding, and thermoforming.
Some examples of thermoplastic polymers include polyethylene (PE), polypropylene (PP), polyvinyl chloride (PVC), polystyrene (PS), and nylon. Each of these polymers has unique properties that make them suitable for specific applications. For instance, PE is widely used in packaging materials due to its excellent moisture resistance, while PVC is often used in pipes and tubing due to its high chemical resistance.
Thermoplastic polymers can also be blended with other materials to modify their properties or reduce their cost. For example, a blend of PP and PE is often used in the automotive industry to make lightweight, durable parts.
Overall, thermoplastic polymers play a significant role in various industries, including automotive, construction, packaging, and electronics, due to their excellent properties and processability.
- Thermosetting Polymers- Also known as thermosets, are a type of polymer that undergoes a chemical reaction to form a rigid, crosslinked network structure. Once formed, these polymers cannot be melted or reshaped by heating, unlike thermoplastic polymers.
The cross-linking process occurs when the polymer is initially formed, typically through a process called curing. During curing, the polymer is heated or exposed to radiation or a chemical catalyst, which causes the polymer chains to react and form strong covalent bonds with one another. These bonds create a rigid, 3D network that is highly resistant to deformation and cannot be melted or reshaped.
Thermosetting polymers have a number of desirable properties, such as high strength, rigidity, and heat resistance. They are commonly used in applications where high temperature and chemical resistance are required, such as in adhesives, coatings, and electronic components.
Some common examples of thermosetting polymers include epoxy resins, phenolic resins, and urea-formaldehyde resins. Epoxy resins are used in a wide range of applications, from coatings and adhesives to composite materials. Phenolic resins are often used in adhesives and molding compounds, while urea-formaldehyde resins are used in adhesives and as binders in wood-based products.
One major disadvantage of thermosetting polymers is that once they are formed, they cannot be recycled or reprocessed. This is because the strong crosslinking bonds that give thermosets their desirable properties also make them difficult to break down and reform. As a result, thermosetting polymers can contribute to environmental waste if not disposed of properly.
The Procedure of Polymerization-
Polymerization is a process that links together small molecules called monomers to form larger molecules called polymers. This process involves three main steps: initiation, propagation, and termination.
- Step 1- Initiation
Initiation is the first step in which an initiator molecule is added to the reaction mixture to begin the reaction. This initiator molecule can be a free radical, a cation, or an anion, depending on the type of reaction. Once the initiator reacts with the monomer, it generates a reactive species like a free radical or a carbocation that can then react with additional monomer molecules.
- Step 2- Propagation
The next step is propagation, where the reactive species formed in the initiation step react with more monomer molecules to grow the polymer chain. This process continues until all the monomers are consumed or until the reaction is terminated.
- Step 3- Termination
Termination is the final step that stops the polymerization reaction. To bring the polymerization process to an end, termination can happen in various ways. This can involve combining two growing polymer chains, the interaction between a polymer chain and a small molecule, or the rearrangement of two reactive species.
The properties exhibited by the final polymer are influenced by factors like the type of monomer utilized, the conditions under which the reaction takes place, and the extent of polymerization. By manipulating these factors during the polymerization process, scientists can deliberately engineer polymers with specific properties to suit diverse applications.
Conclusion
In summary, polymerization is a valuable process that gives us the ability to create materials with unique and remarkable properties. It unlocks a world of possibilities, allowing us to produce a diverse array of substances that can be customized to fulfill a wide range of requirements and overcome various obstacles. The power of polymerization lies in its capacity to shape the characteristics of materials, enabling us to develop innovative solutions and address different needs and challenges.
Polymerization is an important process in the industry, as it allows for the production of materials with tailored properties and structures. Polymers are used in a wide range of applications, including plastics, textiles, adhesives, coatings, and composites. The properties of a polymer can be adjusted by changing the monomer composition, reaction conditions, and post-polymerization treatments, making polymers a versatile and useful class of materials.
|


 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now










