Sublimation DefinitionIntroductionSublimation is a physical process in which a solid substance is transformed into a vapor or gas state without passing through the liquid phase. This process occurs when the substance is heated and the vapor pressure of the solid increases to a point where the molecules can break free from the surface of the solid and enter the gas phase. Sublimation is a type of phase transition and is the reverse process of deposition, where a gas transforms directly into a solid. 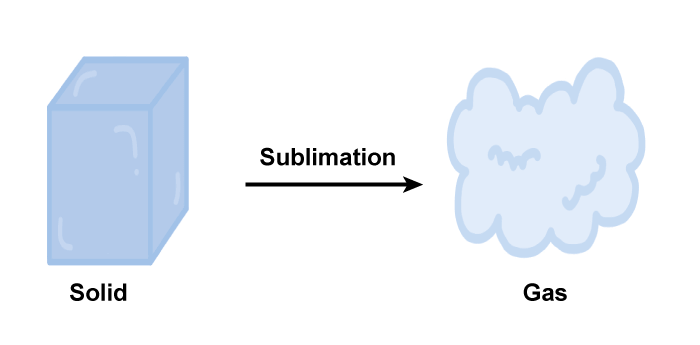
Understanding the Sublimation ProcessThe sublimation process depends on factors such as temperature, pressure, and intermolecular forces within the solid. At a given temperature and pressure, the vapor pressure of a solid determines the ease with which it can sublime. Substances with high vapor pressures tend to sublime more easily than those with low vapor pressures. Sublimation can occur at any temperature below the substance's melting point, but it is more commonly observed at lower temperatures where the energy required to melt the solid is high. Examples of substances that undergo sublimation at room temperature and pressure include dry ice (solid carbon dioxide) and mothballs (naphthalene). The sublimation process can be harnessed for various applications, such as in the purification of substances, the preparation of materials with controlled porosity, and the production of certain drugs and chemicals. Factors Affecting SublimationSeveral factors can affect the sublimation process, including: 1. Temperature Sublimation is typically initiated by applying heat, and the temperature at which a substance sublime depends on the strength of its intermolecular forces. Substances with weaker intermolecular forces will sublime at lower temperatures. 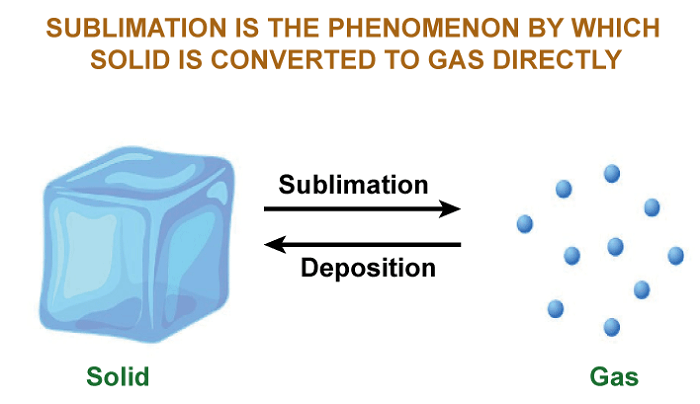
2. Pressure The pressure of the surrounding environment can also affect the sublimation process. At higher pressures, the vapor pressure of the solid substance must be greater to achieve sublimation. 3. Surface Area The surface area of the solid can influence the sublimation rate. A larger surface area provides more space for molecules to escape into the gas phase, which can increase the sublimation rate. 4. Purity Impurities in a solid substance can affect its sublimation properties. Impurities can alter the intermolecular forces within the solid, leading to a change in the sublimation temperature and rate. 5. Crystal Structure The crystal structure of a solid can also affect its sublimation properties. Some crystal structures are more tightly packed than others, affecting the strength of intermolecular forces and the ease with which a substance can sublime. 6. Heating Rate The rate at which heat is applied to the solid can also affect the sublimation process. A slower heating rate can lead to more uniform sublimation. In contrast, a rapid heating rate can result in uneven sublimation and the formation of cracks or other defects in the solid. Types of SublimationThere are three main types of sublimation: 1. Molecular Sublimation The solid substance consists of individual molecules held together by weak intermolecular forces in this sublimation type. Examples of substances that undergo molecular sublimation include iodine, camphor, and naphthalene. 2. Ionic Sublimation This type of sublimation involves the transformation of a solid ionic compound directly into its gaseous state. The process requires a high energy input to overcome the strong electrostatic forces that hold the ions together. Examples of substances that undergo ionic sublimation include ammonium chloride, potassium permanganate, and sodium chloride. 3. Metallic Sublimation This occurs when a metallic substance is heated and transformed directly from a solid to a gas phase. Metallic sublimation is not as common as the other two types and is mainly observed in specific conditions, such as in vacuum or low-pressure environments. Examples of substances that undergo metallic sublimation include cadmium, tungsten, and zinc. Applications of SublimationSublimation has a wide range of applications in various fields, including: 1. Purification of Substances Sublimation can purify certain substances, particularly organic compounds, by separating them from impurities that do not sublime under the same conditions. This process commonly produces drugs, perfumes, and food additives. 2. Drying Sublimation can remove moisture from materials, such as freeze-drying food and pharmaceuticals. 3. Preparation of Materials Sublimation can prepare porous materials with controlled porosity and surface area. Examples include preparing thin films for electronics, ceramics, and metal-organic frameworks (MOFs). 4. Printing Sublimation is used in dye-sublimation printing, in which dyes are printed onto a transfer paper and then transferred to a substrate using heat and pressure. The process is commonly used for printing on fabrics and ceramics. 5. Preservation of Historical Artifacts Sublimation can preserve and restore historical artifacts, particularly those made of delicate or sensitive materials, such as paintings, manuscripts, and textiles. 6. Scientific Research Sublimation is widely used in scientific research for the purification and preparation of materials, particularly for producing high-purity chemicals and samples for spectroscopy and crystallography. 7. Personal Care Sublimation produces personal care products such as deodorants and air fresheners. 8.. Energy Storage Sublimation can store thermal energy in materials such as phase change materials (PCMs), which undergo sublimation and condensation cycles to store and release energy. Sublimation in Everyday LifeAlthough sublimation may not be as obvious in everyday life as other physical changes like melting or boiling, it still has several practical applications that people may encounter: 1. Air Fresheners Many air fresheners use sublimation to release fragrances. The solid material in the air freshener slowly sublimes over time, releasing the fragrance into the air. 2. Dry Ice Dry ice is a form of carbon dioxide that undergoes sublimation at room temperature. It is commonly used as a cooling agent for transporting food, medical supplies, and theatrical fog effects. 3. Freeze-Drying Food Freeze drying is a process that involves the sublimation of water from frozen foods. This process produces instant coffee, fruits, and camping meals. 
4. Mothballs Mothballs are solid naphthalene balls that slowly sublime and release fumes that repel insects. 5. Deodorants Many deodorants use sublimation to release fragrance and antiperspirant agents. The solid material in the deodorant sublimes and releases the active ingredients when applied to the skin. 6. Cleaning Dry cleaning is a cleaning process that involves the sublimation of cleaning agents such as perchloroethylene. The solid cleaning agents are heated and transformed directly into their gaseous state, which can penetrate fabrics and dissolve dirt and stains. 7. Cleaning Computer Keyboards Compressed air dusters use sublimation to clean computer keyboards. The compressed gas cools as it escapes from the can, causing the gas to sublime into a solid and release a stream of gas that can clean the keyboard. 8. Snow and Ice Snow and ice can sublime directly into water vapor when exposed to sunlight or dry air. This process is known as sublimation and is responsible for the gradual disappearance of snow and ice over time, even at temperatures below freezing. Techniques for SublimationSeveral techniques can be used to perform sublimation, depending on the substance being sublimed and the intended application. Here are a few common techniques for sublimation: 1. Vacuum Sublimation Vacuum sublimation involves heating the solid substance in a vacuum chamber to create a low-pressure environment. The reduced pressure allows the substance to sublime without melting. This technique is commonly used for purifying high-purity materials. 2. Gradient Sublimation Gradient sublimation involves creating a temperature gradient along a sample to cause the substance to sublime from one end to the other. This technique separates different components of a mixture based on their sublimation points. 3. Thermal-Gradient Sublimation Thermal-gradient sublimation is similar to gradient sublimation but involves using a thermal gradient instead of a temperature gradient. This technique prepares porous materials with controlled porosity and surface area. 4. Flash Sublimation Flash sublimation involves rapidly heating the solid substance to a high temperature for a short time, causing it to be sublime quickly. This technique is used to prepare thin films and coatings. 5. Chemical Sublimation Chemical sublimation involves reacting a solid substance with a gas to form a volatile compound that can be sublimed. This technique is used to prepare high-purity materials that are difficult to obtain by other means. 6. Microscale Sublimation Microscale sublimation is a technique used to study the sublimation behavior of very small samples, typically on the order of microns or smaller. This technique is commonly used in materials science and nanotechnology research. 7. Freeze-Drying Freeze-drying is a process that involves the sublimation of water from frozen materials, typically using a vacuum chamber. This technique produces high-quality, stable products such as food, drugs, and biological samples. Comparison of Sublimation with Other ProcessesSublimation is a unique physical process with similarities and differences with other processes like melting and boiling. Here are some comparisons between sublimation and other processes: 1. Sublimation vs. Melting Melting and sublimation both involve the transition of a solid to a liquid or gas, respectively. However, melting requires the absorption of heat to overcome the intermolecular forces holding the solid together, while sublimation does not require heat to overcome these forces. Instead, sublimation occurs when the vapor pressure of the solid is greater than the surrounding pressure. 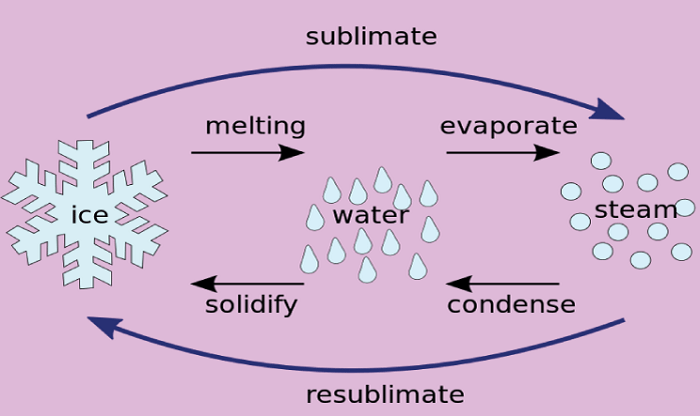
2. Sublimation vs. Boiling Boiling is the process of a liquid changing to gas by adding heat. Unlike sublimation, boiling requires the liquid to reach its boiling point, where its vapor pressure equals the surrounding pressure. Sublimation can occur at temperatures below the boiling point of a substance. 3. Sublimation vs. Deposition Deposition is the reverse of sublimation, where a gas directly changes to a solid. While both processes involve a direct phase transition, deposition occurs when the vapor pressure of the gas is less than the surrounding pressure, causing the gas to condense into a solid. 4. Sublimation vs. Fusion Fusion is the process of combining two or more substances into one, typically involving the melting of a solid and the mixing of liquids. Sublimation, on the other hand, involves the transformation of a solid directly to a gas without mixing or fusion. 5. Sublimation vs. Condensation Condensation is the process of a gas changing to a liquid or solid, typically involving heat removal. While both sublimation and condensation involve phase transitions, sublimation involves the direct transformation of a solid to a gas. In contrast, condensation requires the gas to reach its condensation point, where its vapor pressure equals the surrounding pressure. In summary, while sublimation shares some similarities with other processes like melting and boiling, it is a distinct physical process with unique characteristics and applications. Challenges in SublimationSublimation can present several challenges, especially when achieving high purity or large-scale production. Here are some common challenges associated with sublimation: 1. Temperature Control Sublimation requires precise temperature control to ensure the substance sublimes without melting or decomposing. If the temperature is too high or too low, it can affect the purity and yield of the sublimed material. 2. Pressure control Sublimation also requires precise pressure control to ensure the substance's vapor pressure exceeds the surrounding pressure. If the pressure is too high or too low, it can affect the rate and efficiency of the sublimation process. 3. Contamination During sublimation, it is important to avoid contamination from other substances or impurities in the environment, as this can affect the purity of the sublimed material. Contamination can occur through contaminated equipment, inadequate cleaning procedures, or exposure to air or moisture. 4. Yield and Throughput Sublimation can be a slow process, limiting the yield and throughput of the sublimed material. Large-scale production can require significant time and energy resources, making it difficult to achieve high yields. 5. Equipment and Maintenance Sublimation typically requires specialized equipment, such as vacuum chambers and heating elements, which can be expensive to purchase and maintain. Ensuring the proper operation and maintenance of the equipment is crucial to achieving consistent and reliable sublimation results. 6. Sublimation Point Not all substances can be sublimed easily due to their low sublimation points. Some substances may require a higher temperature or longer processing time to achieve sublimation, which can affect the efficiency and yield of the process. SafetySome substances can be hazardous to handle or release toxic gases during sublimation. Proper safety measures, including ventilation and protective equipment, must be taken to ensure the safety of workers and the environment. Safety Considerations in SublimationSublimation can involve using hazardous materials and equipment, so it is important to take appropriate safety precautions to minimize the risk of accidents and injury. Here are some safety considerations to keep in mind when working with sublimation: 1. Material Safety Some materials used in sublimation can be hazardous or toxic, so it is important to follow proper handling procedures and wear appropriate personal protective equipment (PPE), such as gloves, goggles, and respirators, as necessary. Material safety data sheets (MSDS) should be reviewed to identify potential hazards and appropriate handling procedures. 2. Ventilation Sublimation can release gases and fumes, so ensuring proper ventilation in the work area is important. This can include using fume hoods or local exhaust systems to capture and remove vapors and fumes from the air. 3. Electrical Safety Sublimation equipment typically involves heating elements and electrical components. It is important to ensure that electrical safety protocols are followed, including properly grounding equipment and circuit protection devices. 4. Emergency Procedures In an accident or emergency, it is important to have appropriate procedures, including evacuation procedures and access to emergency equipment, such as fire extinguishers and first aid kits.
Next TopicTeacher Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










