Physical Change DefinitionChanges are happening to everything around us. Chemical or physical changes are both possible. Everything that has weight and occupies space is referred to as matter. It has physical and chemical characteristics and is made up of small particles. The appearance and visible qualities of matter are considered to be its physical attributes. Color, smell, taste, solubility, hardness, fluidity, melting, and boiling points, among other things, are examples of physical qualities. A feature that appears during a chemical reaction is known as a chemical property. A few examples are pH, reactivity, and flammability. Let's examine physical and chemical changes and how they connect to these substances' respective properties. 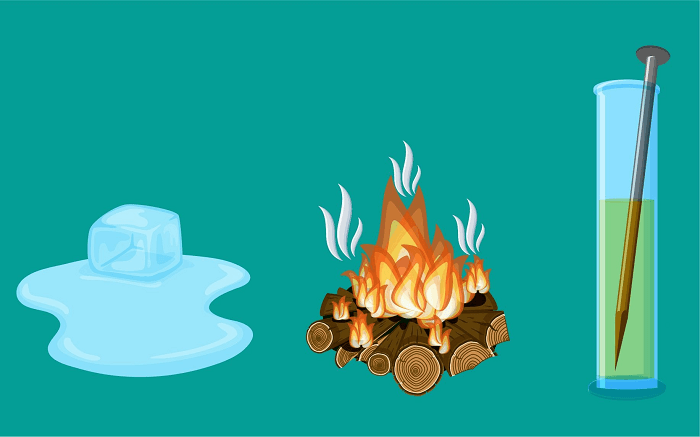
Physical ChangesWe can say that matter has experienced a physical transformation when it changes its visible characteristics. As the physical properties of matter change, there is a type of change known as physical change. Physical changes may affect things like color, smell, solubility, and the state of matter. After a physical transformation, the chemical change or composition of materials is unaffected. The internal composition is unaffected as molecules rearrange themselves throughout this transformation. Physical changes do not affect the chemical property. A Few Examples of Physical Change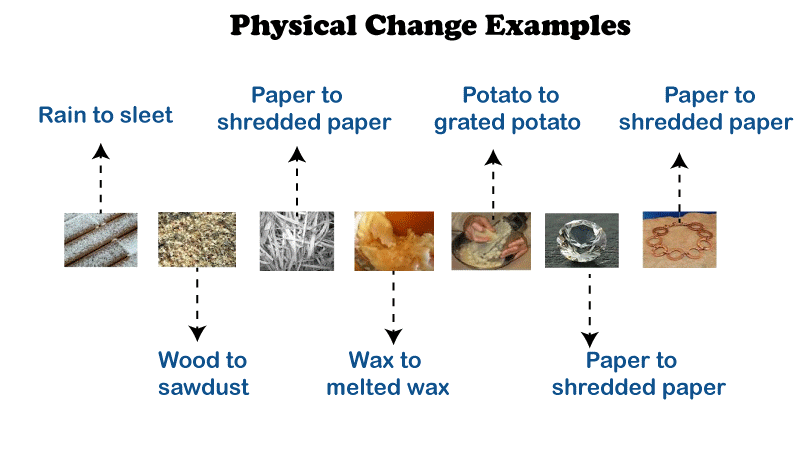
Chemical Change
Chemical changes are changes to the chemical makeup of matter. The word "chemical reaction" is commonly used to describe it. Chemical properties differ between different materials. Depending on this attribute, different compounds have different levels of reactivity. A chemical reaction results in the creation of a brand-new product. The material's composition changes due to breaking molecule bonds during a chemical reaction. Alternatively said, chemical change causes bonds to form and break. Illustrations of Chemical Change
Some instances of chemical change include combustion, corrosion, fermentation, etc. Chemical and physical changes differ from each otherSignificant distinctions between physical and chemical changes are shown in the following. Physical change
Chemical Change
ConclusionA material changes physically when it goes through a physical change and chemically when it goes through a chemical change. No new material is created when something changes physically, but when something changes chemically, a new substance is created. Physical changes are often reversible in nature, but chemical changes are typically irreversible. FAQs: Commonly Asked QuestionsQuestion 1: What physical change has occurred? Answer: A substance undergoes a physical change when a component of its qualities changes, but the substance's identity remains unchanged. Physical changes that are both reversible and irreversible are further divided into two categories. Physically, the process of melting the ice cube may be stopped by refreezing it. Question 2: Which of the following physical changes are current examples? Answer: Materials' composition or size changes are a few examples of physical change. Changing from one state to another, such as from a solid to a liquid or a gas, is considered a physical change. Cutting, bending, dissolving, freezing, boiling, and melting are a few actions that cause physical changes. Question 3: Why are chemical changes different from physical changes? Answer: As something physically changes, its shape or appearance may also change, but its substance does not. On the other hand, a chemical change causes the development of at least one new substance with new properties. Question 4: Does a chemical reaction cause color change? Answer: A color change is another sign that a chemical reaction is occurring. The chemical reaction produced this color change. One must be cautious, though, as a color change can occasionally be caused by blending two colors rather than a real alteration in the makeup of the substances in question. Question 5: Is there a chemical shift when you fry an egg? Answer: As eggs are cooked, the liquid portion turns from liquid to solid, which results in a chemical transformation. At the same time the egg cooks, the liquid portion of the egg turns from transparent to white.
Next TopicPresent Perfect Tense Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










