Base Definition ChemistryWhat is a Base?A chemical molecule known as a base, an alkali, combines with an acid to create salt and water. Bases are frequently employed in several commercial and scientific applications and are generally present in nature. A base in chemistry is a material that can take an extra hydrogen ion (H+) from another chemical and neutralize it. Bases are commonly understood to be substances with a pH higher than 7.0. Ammonium hydroxide (NH4OH), sodium hydroxide (NaOH), and potassium hydroxide are typical bases. Bases are often either non-metallic or metallic compounds. Cations and anions make up metallic bases like barium hydroxide (Ba(OH)2), magnesium hydroxide (Mg(OH)2), and calcium hydroxide (Ca(OH)2). 
On the other hand, molecules like amines, carboxylic acids, and hydroxides make up non-metallic bases. Bases play a crucial role in many industrial processes, including those that produce paper, glass, soap, detergents, and fertilizers. Also, bases are employed in various chemical processes, such as the neutralization of acids, pH regulation, and the catalyzation of certain reactions. They also use medicine to inhibit bacterial growth, neutralize stomach acid, and cure mild skin irritations. In addition, bases are employed to synthesize several organic and inorganic substances. Classification of the BaseStrong and weak bases are the two categories into which bases may be divided.
The pH scale is a logarithmic scale used to quantify the strength of bases. The pH scale ranges from 0 to 14, with seven considered neutral (Neither acidic nor basic). The solution is acidic if its pH value is less than seven and basic if it is more than 7. A base's strength directly relates to its pH; the stronger the base, the higher the pH. Bases can be categorized using the pH scale by looking at their ionization constants. An indicator of how thoroughly a base separates into its ions in an aqueous solution is called an ionization constant, often referred to as a dissociation constant. Strong bases are thought to have large ionization constants, whereas weak bases are considered to have low ionization constants. Finally, bases can be divided into groups based on how reactive they are to other substances. Strong bases are defined as being highly reactive with other compounds, while weak bases are defined as being less reactive with other chemicals. The capacity of a command to take a hydrogen ion from another chemical and initiate a neutralization process determines the base's reactivity. 
To sum up, a base is a chemical substance with a pH greater than seven that can receive a hydrogen ion from another component and produce a neutralization process. The pH, ionization constants, and chemical reactivity of bases are used to categorize them. Bases are crucial for various commercial and scientific methods, including creating multiple inorganic and organic chemicals and paper, glass, soaps, detergents, and fertilizers. Types of the BaseThe base substance that has a pH greater than 7.0. Bases can be classified into two main categories: organic and inorganic.
What is the Natural Base?
Natural bases are chemicals that are basic or alkaline by nature and have an alkaline pH. Biological bases, which are present in living things, are crucial for many chemical processes. Sodium hydroxide (NaOH), potassium hydroxide (KOH), ammonium hydroxide (NH4OH), and borax are examples of natural bases. The pH scale, which measures how basic or acidic a material is, is logarithmic. It is 0 to 14, with seven being neutral. (Neither acidic nor basic). Anything acidic is rated below 7, while anything basic is above 7. A natural base's pH ranges from 8 to 14.
Many chemical processes and reactions depend on natural bases. They are employed in the manufacture of several goods, including food, medicines, and cleaning products. Natural bases are present in living things and have a variety of uses. Numerous chemical processes and reactions would only be feasible with natural bases. How Can We Know if the Substance is Basic or Not?Physical and chemical qualities are the key factors determining whether a material is basic. Physical characteristics include a substance's appearance, texture, sensation, odor, and behavior. Chemical elements describe a substance's chemical makeup and interactions with other substances.
Difference Between Base and Acid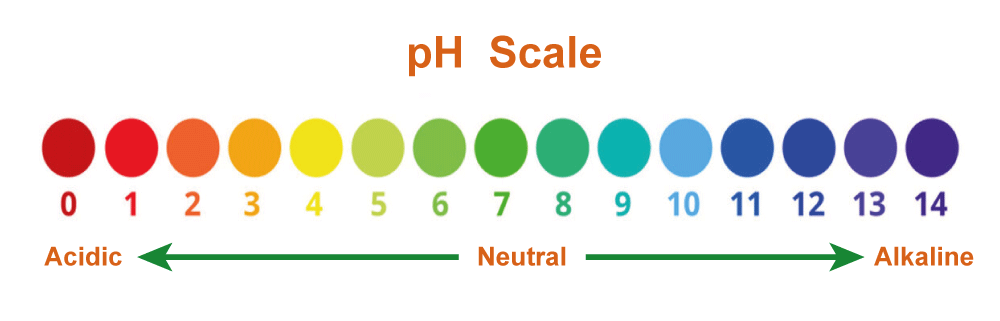
There are several applications for acids' and bases' characteristics in daily life. For instance, many cleaning supplies employ acids and bases to remove stains, oil, and filth. The food business also uses acids to preserve food. In the medical profession, commands treat skin diseases and neutralize stomach acid. Many sectors, including food, medicine, and manufacturing, depend on a substance's acidity or alkalinity. A substance's pH impacts how it interacts with other chemicals and can affect its safety. Acids and bases play various roles in many sectors and are necessary for many commonplace activities.
"Base" Word OriginA category of compounds that react with acids to create salt and water are called bases in chemistry. The word "base" originates in Greek, referring to a low-lying area or the foot of a mountain. The Romans later borrowed this phrase to designate an alkaline material like lime or ash. The word "base" wasn't used scientifically until the 1700s. Chemists were starting to investigate the characteristics of acids and bases to distinguish and categorize them. Joseph Priestley was one of the first researchers to accomplish this, defining a base as "any substance that neutralizes an acid and produces an alkali." Priestley's original definition was elaborated upon by the French scientist Antoine Lavoisier, who said that a base is "any substance that can neutralize an acid and produce a salt." Even though contemporary chemists have a far better understanding of the idea of bases, this terminology is still used today. A list of compounds that may be used to categorize and categorize bases was described as an "alkaline series" by Swedish scientist Jöns Jacob Berzelius in the early 19th century. Alkali, earth, and metallic bases are the three categories Berzelius used to categorize substances. Since then, the word "base," which describes the chemicals that react with acids to form salt and water, has become a crucial chemistry component. Bases are utilized in various chemistry-related operations, including extraction, purification, and neutralization reactions. Today, the word "base" is often used in chemistry and is a crucial component of the vocabulary used in science. It was first used to describe alkaline substances by the ancient Greeks and Romans, and it is still used to define a class of compounds that may neutralize acid and create a salt. Some Most Famous Base, and Acid Names and Its Formula
Which is the Most Famous Reaction of the Base?Neutralization Reaction: Salt and water are produced in this reaction between an acid and a base. For instance, the combination of sodium hydroxide (NaOH) with hydrochloric acid (HCl) results in the production of salt (NaCl) and water. (H2O). The following equation represents this reaction: HCl + NaOH → NaCl + H2O
Next TopicBeer-Lambert Law Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










