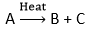Decomposition Reaction DefinitionThe process of breakdown of any physical or chemical entity is referred to as decomposition. Chemical decomposition results from breaking down a complex chemical entity into one, two, or more components. Decomposition Types1. Physical Decomposition or Changes Physical changes are typically reversible. However, they can also be irreversible and only affect the physical characteristics. A substance's physical characteristics modify when it goes through a physical change. For example, breaking glass. 2. Chemical Decomposition or Changes Chemical changes are permanent transformations in which new products are created. The reactant and product are different entity and has completely distinct characteristics. Chemical equations are a simple way of representing a chemical reaction. Chemical equations act as a symbolic representation of the chemical reaction. 
Decomposition ReactionA decomposition reaction is a chemical process in which one reactant splits into more than 1 item. Most of the time, the decomposition reactions need energy. One of its main uses is the extraction of metals from ores. Decomposition Reactions Types1. Thermal Decomposition It is a decomposition in which a single reactant is split into several products with heat. For thermal decomposition processes, high temperatures are required. For e.g.: CaCO3 → CaO + CO2 2. Photo Decomposition This decomposition reaction uses light or photons to split the reactants into several products. The reactant's bonds are broken by utilizing light energy. Photolysis is another name for this reaction. For e.g.: 2AgCl → 2Ag + CL2 3. Electrolytic Decomposition Electrolytic decomposition refers to the breakdown of any molecule using electricity. Certain chemicals quickly separate when heated by electricity. For e.g., Electrolysis of water. 2H2O → 2H2 + O2 Application of Decomposition Reactions
Double Decomposition ReactionIn a double decomposition reaction, two reactants exchange negative and positive ions to create two new molecules. For example, HCl(aq) + NaOH(aq) →NaCl(aq) + H2 O (l) Here,
FAQs on Decomposition Reaction1. Difference between combination reaction and decomposition reaction Answer:
2. Are all decomposition processes endothermic in nature? Answer: Yes, practically all decomposition processes require heat to break down; as a result, they are endothermic in nature. 3. Differentiate between endothermic and exothermic reactions. Answer:
4. Is decomposition a chemical or physical process? Answer: The chemical compound splits into tiny particles during decomposition. Thus, it is a chemical process instead of a physical one. 5. List the main applications of the decomposition reaction. Answer: Some significant applications of the decomposition reaction include the following:
6. List examples of endothermic processes. Answer:
7. What are the applications of endothermic reactions? Answer: Here are a few applications for it: 1. Photosynthesis Unlike mammals (including humans), trees make their food. Photosynthesis is the procedure by which trees generate food, which is thought to result from an endothermic reaction. Photosynthesis involves sunlight absorption through leaves and producing food when mixed with water and carbon dioxide. 2. Sublimation The process of sublimation is another illustration of an endothermic reaction. In sublimation, a substance can move from the solid to the gaseous. For e.g., dry ice, dry ice is simply solid carbon dioxide. A molecule directly transitions from a liquid to a gas state in evaporation. 3. Cooking food An endothermic reaction occurs during the cooking of food. Let's imagine that you are making noodles. The noodles take in the heat from the pan as it cooks. 8. What are the applications of decomposition reactions? Answer: Here are a few applications for it: 1) Fertiliser Production By utilizing the decomposition reaction, fertilizers are produced. Nitrogen gas ( ) and water are produced when ammonium nitrate decomposes. Trees utilize nitrogen gas for growth, and water aids in photosynthesis. 2) Food Preserving Nitric oxide and nitrogen dioxide are produced during the decomposition of sodium nitrite ( , which works as a preservative in meat products. 3) Pollutant Removal Pollutants are removed from the environment using decomposition processes. 8. Differentiate thermal decomposition and thermal dissociation Answer:
9. What are the similarities between thermal decomposition and thermal dissociation? Answer: A compound can be divided into smaller components utilizing heat energy during thermal decomposition as well as thermal dissociation. 10. What is decomposition? Answer: The procedure of breaking down or condensing any physical or chemical substance. Decomposition Types 1) Physical Decomposition Although physical changes are typically reversible, they can also be permanent and only affect the physical properties. A substance's physical qualities alter when it goes through a physical change. For, e.g., breaking glass, etc. 2) Chemical Decomposition Chemical transformations are permanent transformations in which new products are created. 11) What is the mean by electrolytic decomposition? Answer:
12) Explain the reversible reaction Answer: In a reversible reaction, the reactants create products that later combine to return the reactants to their original state. When a reversible reaction reaches equilibrium, the concentrations of the reactants and products stop changing. NH4 Cl(s) → NH3 (g) + HCl(g)
Next TopicGas Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share