Molarity Formula and DefinitionMolarity is a unit of concentration used to express the amount of a solute dissolved in a solution. It is defined as the number of moles of solute per liter of solution. In simpler terms, molarity measures the concentration of a solution in terms of the number of particles (molecules or ions) that are dissolved in a given volume of the solvent. 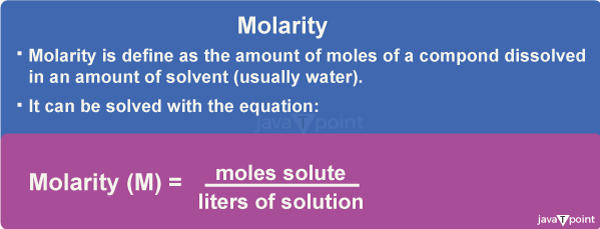
Molarity FormulaThe molarity formula can be written as: Molarity (M) = moles of solute/liters of solution Or, Molarity (M) = mass of solute (in grams) / molar mass of solute (in g/mol) x volume of solution (in liters) where,
To calculate molarity, we need to know the amount of solute in moles and the volume of the solution in liters. We can also use the mass of the solute and its molar mass to calculate the number of moles and then use the molarity formula to calculate the molarity of the solution. Molarity ExampleSuppose we have 10 grams of sodium chloride (NaCl) dissolved in 1 liter of water. To calculate the molarity of this solution, we need to know the molar mass of NaCl, which is 58.44 g/mol. First, we need to calculate the number of moles of NaCl in the solution: Number of moles of NaCl = mass of NaCl / molar mass of NaCl = 10 g / 58.44 g/mol = 0.171 moles Now we can use the molarity formula to calculate the molarity of the solution: Molarity (M) = moles of solute/liters of solution = 0.171 moles / 1 liter = 0.171 M Therefore, the molarity of the sodium chloride solution is 0.171 M. Molarity vs. MolalityMolarity and molality are both units of concentration, but they differ in the way they measure concentration. Molarity measures concentration in terms of the number of moles of solute per liter of solution, while molality measures concentration in terms of the number of moles of solute per kilogram of solvent. Molality is a more accurate measure of concentration because it takes into account the mass of the solvent, which not changes with temperature or pressure. Molarity, on the other hand, assumes that the volume of the solution that changes with change in temperature or pressure. Molarity vs. NormalityNormality is another unit of concentration used in chemistry. It measures the concentration of a solution in terms of the number of equivalents of solute per liter of solution. An equivalent is a measure of the number of reactive species (ions or molecules) in a solution that can react with other substances. Normality is used in acid-base chemistry, where the concentration of a solution is measured in terms of the number of acid or base equivalents present in the solution. For example, a solution of hydrochloric acid (HCl) with a normality of 1 N contains one mole of H+ ions per liter of solution. The molarity and normality of a solution can be related by the following equation: Normality (N) = molarity (M) x n where, n is the number of valence electrons (number of reactive species). The value of "n" depends on the type of reaction being studied. For acid-base reactions, n is equal to the number of acidic or basic protons that can be donated or accepted by the solute. For example, for a monoprotic acid such as HCl, n is equal to 1, while for a diprotic acid such as H2SO4, n is equal to 2. Molarity ApplicationsMolarity is a very useful concept in many areas of chemistry, including analytical chemistry, biochemistry, and physical chemistry. Some common applications of molarity are:
Units of MolarityThe unit of molarity is moles per liter (mol/L). This unit is also known as a molar or M. Therefore, a 1 M solution contains 1 mole of solute per liter of solution, while a 0.5 M solution contains 0.5 moles of solute per liter of solution. Other units of concentration used in chemistry include:
Limitations of Molarity
ConclusionIn conclusion, molarity is a fundamental concept in chemistry that is used to measure the concentration of a solution in terms of the number of moles of solute per liter of solution. The molarity formula can be used to calculate the molarity of a solution, and it is an important tool in many areas of chemistry. By understanding the concept of molarity, chemists can make accurate calculations and predictions about chemical reactions and solutions, and develop new and improved chemical formulations. It is a versatile tool that is used in a variety of applications, including the preparation of solutions, stoichiometry calculations, titrations, pharmaceutical formulations, and electrochemistry experiments. Molarity has its limitations, and it is important for chemists to understand these limitations when using molarity to describe solutions. Despite its limitations, molarity remains an essential concept in chemistry, providing a powerful tool for chemists to understand and control the properties of solutions and chemical reactions.
Next TopicOhm's Law Definition Class 10
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










