Hydrolysis DefinitionWater serves as one of the reactants in a hydrolysis reaction, and it is typically used to break down chemical bonds in the other reactant. People might think of hydrolysis as the reverse of a condensation reaction, which occurs when two molecules unite and one of the products is water. The Greek prefix hydro- (meaning "water") and the verb lysis (meaning "to separate") are the word's sources. 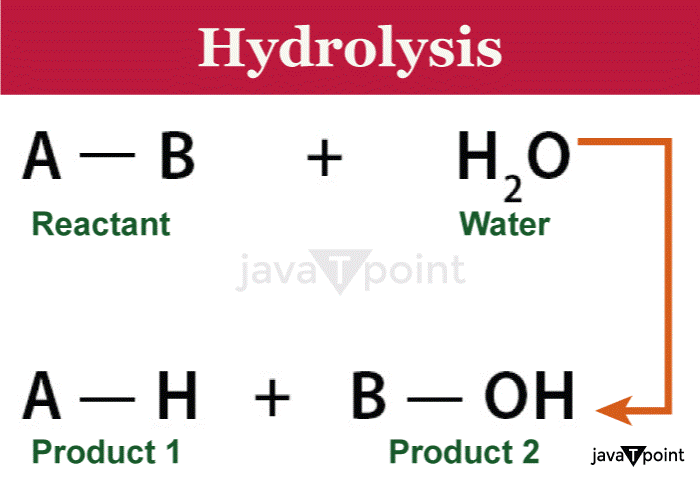
A hydrolysis procedure's general formula is: AB + H2O => AH + BOH Water and an ester react during organic hydrolysis processes. RCO-OR' + H2O => RCO-OH + R'-OH History of HydrolysisSince the beginning of civilization, when people first learned about the properties and reactions of various substances, hydrolysis has been a common process. But in the late 18th and early 19th centuries, a methodical study and comprehension of the chemical process of hydrolysis began to take shape. One of the earliest developments in the understanding of hydrolysis was the work of Swedish scientist Torbern Bergman in the middle of the 18th century. Bergman was aware that the interaction of water with various substances led to the dissolution of compounds and the synthesis of new molecules. His discoveries served as a springboard for further investigation into the process of hydrolysis. The understanding of hydrolysis has greatly benefited from the research done by French scientist Louis Jacques Th�nard at the start of the 19th century. Th�nard conducted a great deal of research into the ways in which water interacts with different compounds, including acids and salts. He realised that these substances might be disassembled by water into their individual ions or molecules, leading to the synthesis of new compounds. Another key development in hydrolysis history was made possible by the work of German scientist Friedrich W�hler. In 1828, W�hler synthesised urea by combining ammonium cyanate and water, an organic compound found in urine. This discovery challenged the prevalent vitalism paradigm, which maintained that only living creatures could synthesize organic compounds. W�hler's production of urea by a non-biological process demonstrated the importance of hydrolysis in the synthesis of organic compounds. In the 19th and 20th centuries, organic chemistry advanced, and with it, our knowledge of hydrolysis. Chemists began examining in greater depth the precise mechanisms and environmental elements that affect how organic molecules are hydrolyzed. They looked at the impact of temperature, pH, and catalyst presence on the rate and result of hydrolysis reactions. The concept of hydrolysis in organic chemistry is well-illustrated by the hydrolysis of esters. Charles-Adolphe Wurtz, a French scientist, developed a method for hydrolyzing esters in the late 19th century that used acids or bases as catalysts. This procedure, called saponification, resulted in the production of carboxylic acids and alcohols from ester molecules. Since then, the saponification process has been used more and more in the manufacture of soap and detergent. Since the establishment of enzymology in the 20th century, scientists have been investigating how enzymes work to catalyze hydrolysis reactions in biological systems. With the aid of specific enzymes, such as amylases, lipases, and proteases, proteins, lipids, and carbohydrates can all be hydrolyzed. Research on enzyme-mediated hydrolysis offered light on the operation and unique characteristics of these vital biological processes. Our understanding of hydrolysis has advanced recently because of the creation of new analytical tools and computational techniques. Scientists have been able to get insight into the atomic-level complexities of hydrolysis reactions through computer simulations and molecular modelling. Additionally, the products and intermediates of hydrolysis processes may now be identified and characterized thanks to current spectroscopic techniques like nuclear magnetic resonance (NMR) and mass spectrometry. Common Examples of HydrolysisHydrolysis was used to make soap for the first time in a commercial environment. When a triglyceride (fat) is mixed with water and a base, usually sodium hydroxide (NaOH) or potassium hydroxide (KOH), soap is created by the hydrolysis of the base. Fatty acids and bases interact to produce glycerol and salts, which later transform into soap. Types of Hydrolysis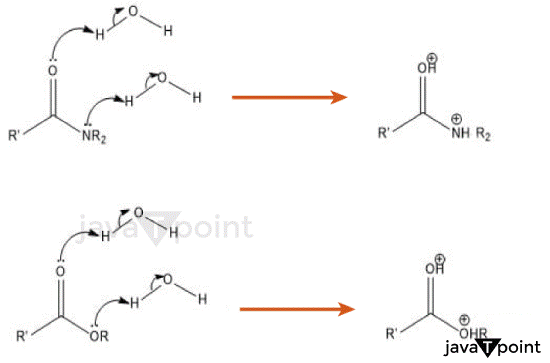
Acidic Hydrolysis: When an acid is used as the hydrolyzing agent, acidic hydrolysis happens. The conjugate base of the molecule and a positively charged hydroxonium ion (H3O+) are created when the acid gives a proton to the compound. Following its interaction with the molecule, this ion breaks the covalent bonds and creates new products. The digestion of food in the stomach is one of the most typical instances of acidic hydrolysis. Complex molecules like proteins, polysaccharides, and lipids are broken down into simpler parts that the body can absorb by the gastric acid, which is mostly made of hydrochloric acid (HCl). Basic Hydrolysis: When the hydrolyzing agent is a base, basic hydrolysis?also referred to as alkaline hydrolysis?occurs. The molecule receives a hydroxide ion (OH-) from the base, which causes the conjugate acid and a negatively charged hydroxide ion to form. When this hydroxide ion interacts with the chemical, the covalent bonds are broken and new products are created. The saponification of esters is a prominent example of basic hydrolysis. Esters are hydrolyzed into alcohol and a carboxylate ion by strong bases like sodium hydroxide (NaOH). This method is used to create soaps and detergents, in which fats and oils are saponified to create the appropriate salts of fatty acids (soaps). Enzymatic Hydrolysis: Enzymes are utilised during enzymatic hydrolysis to catalyse hydrolytic processes. Chemical reactions in living things are sped up by enzymes, which are biological catalysts. By reducing the energy needed for the process to start, they hasten the hydrolysis of particular molecules. In numerous biological processes, such as digestion, cellular metabolism, and the dissolution of complex compounds, enzyme hydrolysis is frequently used. For instance, the enzyme amylase, which is found in saliva and pancreatic secretions, converts starch into less complex carbohydrates like maltose and glucose. The breakdown of fats into glycerol and fatty acids is aided by lipases, which also make it easier for the body to absorb and use these fats. Photolytic Hydrolysis: When light energy triggers the hydrolysis reaction, it is said to be photolytic hydrolysis. In the presence of water molecules, certain substances can go through a process called photolysis whereby they take in photons and then destroy their covalent bonds. Photolytic hydrolysis reactions are frequently started using ultraviolet (UV) light. The destruction of ozone (O3) in the atmosphere of the Earth is a case of photolytic hydrolysis. Ozone molecules split apart into reactive oxygen species (ROS) and oxygen (O2) as a result of solar UV radiation, which adds to the natural ozone cycle. Hydrolysis in Biomolecules
Triglycerides, which have a glycerol backbone and three fatty acid molecules attached, are the most prevalent type of lipid. To create individual fatty acids and glycerol molecules, triglycerides must be hydrolyzed. During this process, which primarily takes place in the small intestine, an enzyme called pancreatic lipase catalyses the hydrolysis of triglycerides in the presence of water. The hydrolysis of triglycerides yields glycerol and fatty acids, which can then be absorbed by intestinal cells. Once within the cells, these substances can be used to either produce new lipids or go through extra metabolization to provide energy. Another prominent lipid class that is present in substantial amounts in cell membranes is phospholipids. They are composed of two fatty acids, a phosphate group, two additional molecules, and a glycerol molecule connected to them. During the hydrolysis of phospholipids, ester bonds that hold the fatty acids to the glycerol backbone are broken, resulting in the formation of fatty acids, glycerol, and phosphate groups.
Hydrolysis in Esters- Chemical ProcessEsters are a family of organic substances that are widely employed in many sectors of the economy, including the food, fragrance, and pharmaceutical industries. Their distinctive flavours and smells are a result of their special chemical makeup. Esters can, however, undergo hydrolysis, a chemical event that breaks down ester bonds in the presence of water or hydroxide ions. Esters are not eternally stable. The mechanism of esters' hydrolysis, as well as its applications in various disciplines, will all be discussed in this essay. Esters undergo hydrolysis when water molecules attack the carbonyl carbon and break the ester bond, resulting in the formation of a carboxylic acid and an alcohol. Acid-catalyzed hydrolysis and base-catalyzed hydrolysis, respectively, are reactions that are commonly catalysed by an acid or a base. Both reactions adhere to separate pathways. In acid-catalyzed hydrolysis, an acid proton gives oxygen to the ester, resulting in the formation of a tetrahedral intermediate. When the tetrahedral intermediate collapses, an alcohol and a protonated carboxylic acid are produced. The desired carboxylic acid is then produced as a result of deprotonation. To turn esters into carboxylic acids for subsequent chemical transformations, this process is frequently used in laboratory settings. Contrarily, in base-catalyzed hydrolysis, a hydroxide ion attacks the carbonyl carbon of the ester, resulting in the creation of a negatively charged tetrahedral intermediate. The intermediate then loses a hydroxide ion, resulting in the formation of the matching carboxylic acid and alcohol. Industrial operations like the saponification of fats and oils to make soap frequently use base-catalyzed hydrolysis. Acidic Hydrolysis of Esters: R-COOR' + H2O ⇌ R-COOH + R'-OH Where R and R' represent alkyl or aryl groups. For example, Ester ethyl acetate (CH3COOCH2CH3) undergoing acidic hydrolysis with sulfuric acid (H2SO4), the reaction would be: CH3COOCH2CH3 + H2O ⇌ CH3COOH + CH3CH2OH This reaction produces acetic acid (CH3COOH) and ethanol (CH3CH2OH) as the products. Basic Hydrolysis of Esters: R-COOR' + H2O ⇌ R-COOH + R'-OH Where R and R' represent alkyl or aryl groups. For example, ester ethyl acetate (CH3COOCH2CH3) undergoing basic hydrolysis with sodium hydroxide (NaOH), the reaction would be: CH3COOCH2CH3 + H2O ⇌ CH3COOH + CH3CH2OH This reaction also produces acetic acid (CH3COOH) and ethanol (CH3CH2OH) as the products.
Esters have numerous important uses that can be achieved through hydrolysis. Enzymatic hydrolysis of esters is one method used in the food business, for instance, to create artificial flavourings. A group of enzymes called lipases can selectively hydrolyze the esters present in natural sources, producing the flavours and fragrances that are sought after. Esters' hydrolysis is a crucial step in the metabolism of drugs, according to the pharmaceutical industry. Esters are frequently employed in prodrugs, which are essentially inactive substances that the body hydrolyzes to produce active drugs. Improved drug distribution and higher bioavailability are made possible by the controlled hydrolysis of ester prodrugs. Hydrolysis of AmidesAmides are a class of organic compounds that contain a carbonyl group (C=O) bonded to a nitrogen atom. They are widely found in nature and have important roles in biological processes, such as protein synthesis. However, amides can undergo hydrolysis under specific conditions, a chemical reaction that breaks the amide bond and transforms the amide into a carboxylic acid and an amine. In this essay, we will delve into the hydrolysis of amides, its mechanisms, and its significance in various fields. The hydrolysis of amides is a chemical reaction that involves the cleavage of the amide bond in the presence of water or hydroxide ions. This reaction leads to the formation of a carboxylic acid and an amine. The hydrolysis of amides can occur through acid-catalyzed or base-catalyzed mechanisms, each following distinct reaction pathways. In acid-catalyzed hydrolysis, a strong acid, such as hydrochloric acid or sulfuric acid, is used as a catalyst. The acid donates a proton to the nitrogen atom of the amide, resulting in the formation of an ammonium salt intermediate. This intermediate is unstable and readily undergoes further hydrolysis. The water molecule attacks the carbonyl carbon of the ammonium salt, breaking the amide bond and forming a tetrahedral intermediate. The tetrahedral intermediate subsequently collapses, resulting in the formation of a carboxylic acid and an ammonium ion. Finally, the ammonium ion can be converted back to an amine by deprotonation. The overall reaction can be represented as follows: RCONH2 + H2O → RCOOH + NH4+ In base-catalyzed hydrolysis, a strong base, such as sodium hydroxide or potassium hydroxide, is used as a catalyst. The hydroxide ion attacks the carbonyl carbon of the amide, resulting in the formation of a tetrahedral intermediate with a negatively charged oxygen atom. This intermediate readily undergoes further hydrolysis. The water molecule attacks the carbonyl carbon of the tetrahedral intermediate, breaking the amide bond and forming a carboxylate ion and an amine. The overall reaction can be represented as follows: RCONH2 + OH- → RCOO- + NH2- Advantages & Disadvantages of HydrolysisAdvantages
Disadvantages
Next TopicMafia- Definitive Edition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










