Ductility DefinitionThere are numerous elements, metals, and non-metals around us; few of them have been discovered by scientists, and there are many to be discovered; students from a science background can easily relate to the topic, but those who are not from a scientific background and those who don't have adequate knowledge regarding the properties and special features of metals and non-metals, even they can easily relate the topic in this article. There are various metals and non-metals around us, and they possess some physical and chemical properties that make them unique from others. Their properties make them useful for society because they help in solving various problems, and at the same time, they deduct the overall cost of production. In this article, we discuss "Ductility," which is one of the physical properties of some natural metals found in the nature. What is Ductility?As mentioned above, this is the physical property found in metals that help them to stretch until it reaches it's breaking point, unlike other metals that break down into pieces even if a small amount of Force is applied over them. If metal is malleable, applying the Force of a given newton (SI unit of Force) on metal will expand rather than break it into pieces. If a metal has the property to become ductile, then we can mold it into a thin wire by stretching and beating. 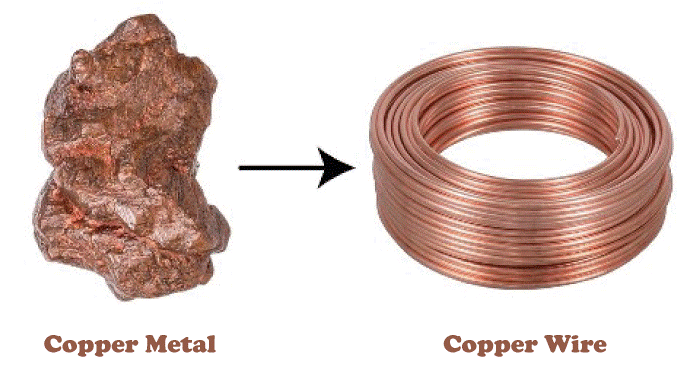
As it is clear from the image shown above what ductility is and what it looks like, in the given image, there is a metal (Copper) that is extracted in its raw form that is not at all thin and appears like a wire, on the other side of the image we saw a wire is made from the same metal that is shown on the left side, this is possible just because that element possesses ductility property, and when it is hammered or beaten then rather than breaking it transform into thin wire, that can be used in houses and industries to conduct electricity. If metal is ductile, it doesn't mean we can transform it into a wire of our desired length because every metal has a fixed breaking point. If we apply a force (in Newton) greater than their breaking point, the metal will break down into pieces; unlike other metals, every metal has its different breaking point. A few examples of metals that possess Ductility properties along with their breaking points are as follows:
What is Malleability?We are also discussing "Malleability" in this article because there is a misconception regarding this property in many students, many students think that both Malleability and Ductility are similar and if a metal is Ductile, then it must be Malleable also, but this is completely wrong because both are different. However they both are the physical property of metals, but they are different in terms of the Force applied to them; if a metal possesses the Malleability property, then the Force applied over it is compressive stress; on the other hand, the Force applied on Ductile metals are Tensile stress, the only difference in both types of stress is that the compressive stress transforms the metal into a sheet. It can be folded easily into a roll; on the other side, tensile stress transforms the metal into foldable wire. A few examples of metals that are malleable are as follows:
Real-Life Application of Metals Having this PropertyThere are various practical applications of metals that possess "Ductility" that are mentioned as follows:
Next TopicPrinter Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share









